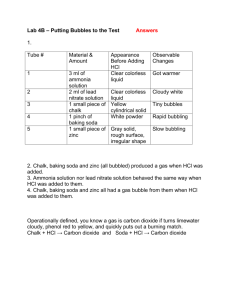
Life Sciences 1a An Integrated Introduction to the Life Sciences
advertisement
The Value of Active Learning: Understanding Acidity Group 2 Sarah Malmquist Joanna Kiryluk Stony Brook University Stony Brook University Chandrika Narayan Khaliliah Reddie University of Massachusetts, Lowell University of Massachusetts, Lowell Valerie Purdie-Vaughns Brent Stockwell Columbia University Columbia University Learning goals • Appreciate active learning vs traditional teaching strategies • Understand what pH is and be able to determine pH of sample Learning objectives You should be able to… Distinguish between active learning and traditional lectures Define pH Determine the pH of a solution using litmus paper Calculate the pH of a solution given the proton concentration Setting the scene Problem to solve: readiness for active learning Who are you? • Students in Bio 101 (or any introductory science class) • First day of class What will you do? • Participate in two activities Part 1 Lecture: acidity Acidity is a critical feature of biological systems • Acidity is the concentration of H+ – measured using pH • pH is important living systems – fish in water – human blood – tumor growth – digestion pH measures the concentration of protons in a solution The pH typically varies from 0 to 14 [H+] = concentration of H+ in Molarity (M = moles/Liter) pH = - log [H+] How does the Log function work? Log 10 = Log (101) = 1 Log 100 = Log (102) = 2 Log 1000 = Log (103) = 3 Log 0.1 = Log (10-1) = -1 The log (base 10) of a number is the exponent the number 10 needs to be raised to in order to generate that number e.g. 0.001 M H+ = 10-3 M H+ = pH of 3 pH 3 = 10-3M Acids have removable protons that dissolve in solution • Lower pH = higher [H+] = acidic • Lemon juice = pH 2 If [H+] = 10-7 M, pH = 7 = neutral water H2O H+ + OH- [H+] in water = 10-7 Solutions that differ by 1 pH unit have a ten fold difference in [H+] Lemon Juice pH = 2 0.01 M H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ Vinegar pH = 3 0.001 M H+ H+ H+ H+ H+ H+ H+ H+ H+ H+ Example of a pH calculation Add 1 mL 1M HCl (1 mol/liter) to 1 L water, pH drops from 7 to 3 • • • • • Why? 1 mL of 1M HCl has 10-3 moles of H+ 10-7 mol (0.0000001 mol) in 1 L water (negligible by comparison) [H+] when added to 1 L = 10-3 moles/liter log[10-3] = -3 pH = -log[H+] = 3 Summary • pH is a numerical scale for determining the acidity of a solution • pH = - log[H+] • It is important to know how to calculate changes in the pH of a solution Clicker Question You have 5 mL of a solution of pH 4 and 5 mL of another solution of pH 7. Which of the following is the closest estimate for the resulting pH if you mix the two solutions together? A. 3.5 B. 4.5 C. 5.5 D. 6.5 E. 7.5 Part 2 Active Learning: acidity A hands-on pH experiment GOAL: Understand how to measure and calculate pH 1. How to use a litmus paper to measure pH of a solution. • Litmus paper: a strip of indicator paper that is dipped in a sample liquid. • The paper turns a different color depending upon the pH of the solution. • The strip is compared to a color standard to determine pH A hands-on pH experiment 2. Measurements Use tube : “A” (5 mL of soda), “B” (5 mL of water), “C” (40 mL of water) Measurement 1: •Take a strip of litmus paper and dip the end into a tube A with soda. •Measure the pH with litmus paper, record the pH. •Convert your measured pH to the [H+] in the sample and record it Measurement 2: •Pour water from tube B to soda in tube A. •Now you have diluted the original soda 2-fold. •Use a strip of litmus to measure the pH of the diluted soda •Record the pH, convert it to the [H+] in the sample, and record it Measurement 3: •Pour water from tube C to tube A to further dilute the soda. •Now you have diluted the original soda 10-fold. •With a new strip of litmus paper, measure the pH. •Convert your measured pH to the [H+] in the sample and record it A hands-on pH experiment pH = –log [H+] Soda volume (mL) Water volume (mL) Volume Fold (soda+wat dilution of er) soda (mL) 1. 5 0 5 2. 5 5 10 3. 5 45 50 pH [H+] (M) 1 • Please label each pH strip after you use it • What was the pH change after a two-fold dilution? • After a 10-fold dilution? Clicker Question You have 5 mL of a solution of pH 4 and 5 mL of another solution of pH 7. Which of the following is the closest estimate for the resulting pH if you mix the two solutions together? (Talk with your whole table.) A. 3.5 B. 4.5 C. 5.5 D. 6.5 E. 7.5 Clicker Question You have 5 mL of a solution of pH 4 and 5 mL of another solution of pH 7. Which of the following is the closest estimate for the resulting pH if you mix the two solutions together? A. 3.5 B. 4.5 C. 5.5 D. 6.5 E. 7.5 SOLUTION • [H+] = 10-4 M = 0.00010 M • -log[10-4] = 4 = pH • The number of protons in water is 10-7 M (0.0000001 M), which is negligible by comparison • Two-fold dilution of the pH 4 solution • [H+] = 5 x 10-5 = 0.00005 • -log(5x10-5) = 4.3 = pH of mixture Brainstorm • How did the hands-on experiment help prepare you for the clicker question? • Complements lecture • Adds excitement Decreased failure rate Freeman et al., 2014, Proceedings in the National Academy of Sciences “I need the best possible grade in this course” Active learning in class Higher grades Dream come true! Learning goals realized… You should now have… an appreciation for active learning compared to traditional teaching An understanding of what pH is and the ability to determine the pH of a solution Thank you. Results of clicker question after lecture Results of clicker question after hands-on activity Supplemental handout Example of a pH calculation If you add 1 mL of 1M HCl (1 mol/liter) to 1 liter of water, the pH of the water drops from 7 to 3 Solution for people who “think in equations” (include physicists, mathematicians, etc) and want to be able to calculate pH in the most general case V = volume, éë H + ùû = concentration Vtotal ´ éë H + ùû = VHCl ´ éë H + ùû + Vwater ´ éë H + ùû final HCl water mol +ù -3 é Vtotal ´ ë H û = 10 L ´1 + final L Concentration: 10 -3 mol mol 1L ´10 L -7 10-7 is negligible, compared with 10 -3 mol éë H + ùû » VHCl ´ éë H + ùû = 10 -3 ´1M final HCl Vtotal pH: pH final æ + ö V = - log10 çéë H ùû ´ HCl ÷ = - log10 (10 -3 ) = 3 HCl Vtotal ø è TITLE • TEXT