Practice problems for EXAM 2
advertisement
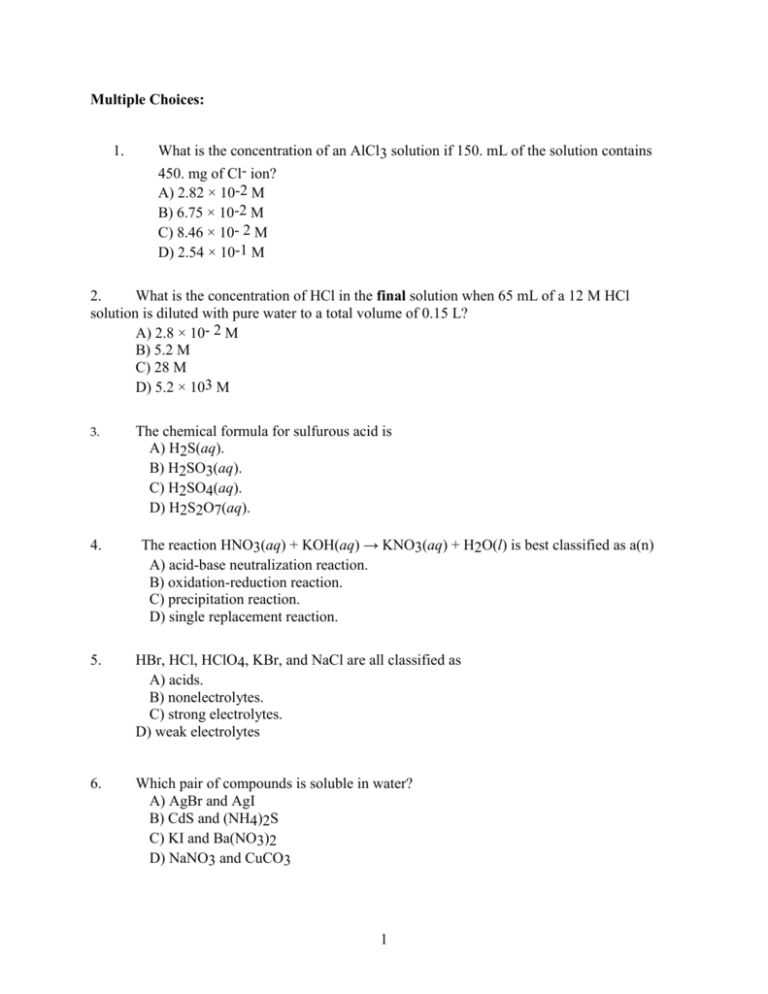
Multiple Choices: 1. What is the concentration of an AlCl3 solution if 150. mL of the solution contains 450. mg of Cl- ion? A) 2.82 × 10-2 M B) 6.75 × 10-2 M C) 8.46 × 10- 2 M D) 2.54 × 10-1 M 2. What is the concentration of HCl in the final solution when 65 mL of a 12 M HCl solution is diluted with pure water to a total volume of 0.15 L? A) 2.8 × 10- 2 M B) 5.2 M C) 28 M D) 5.2 × 103 M 3. 4. The chemical formula for sulfurous acid is A) H2S(aq). B) H2SO3(aq). C) H2SO4(aq). D) H2S2O7(aq). The reaction HNO3(aq) + KOH(aq) → KNO3(aq) + H2O(l) is best classified as a(n) A) acid-base neutralization reaction. B) oxidation-reduction reaction. C) precipitation reaction. D) single replacement reaction. 5. HBr, HCl, HClO4, KBr, and NaCl are all classified as A) acids. B) nonelectrolytes. C) strong electrolytes. D) weak electrolytes 6. Which pair of compounds is soluble in water? A) AgBr and AgI B) CdS and (NH4)2S C) KI and Ba(NO3)2 D) NaNO3 and CuCO3 1 7. What reagent would distinguish between Ba2+ and Pb2+? A) NaCl B) NaNO3 C) Na2SO4 D) Na2S2O3 8. Which of the following instruments directly measures the pressure of a gas? A) spectrometer B) manometer C) polarimeter D) gas chromatograph 9. Which one of the following is not used to describe the condition of a gas? A) number of moles B) polarity C) temperature D) volume 10. When temperature-volume measurements are made on 1.0 mol of gas at 1.0 atm, a plot V versus T results in a A) hyperbola. B) parabola. C) sine curve. D) straight line. 11. One mole of which gas has the greatest density at STP? A) Ar B) N2 C) CO D) All three gases have the same density. 12. Some assumptions from the kinetic molecular theory are listed below. Which one is most frequently cited to explain compressibility of a gas? A) The average kinetic energy of gas particles is proportional to the Kelvin temperature. B) Collisions of gas particles are elastic and total kinetic energy of the gas is constant. C) A gas consist of tiny particles moving in random straight line motion. D) The volume of the particles is negligible compared to the volume of the gas 2 QUESTION II A. Provide the oxidation number for the designated atom(s) in each of the compound. Please report the oxidation number of the individual atom SnF2 Sn = F= CO32- C= O= B. P4O6 P= O= Using the activity series, determine whether the following redox reaction will occur (go forward). If no, write NR (no reaction). If yes, finish the reaction and identify the reducing agent, oxidizing agent and write the half reaction for each *Must show the oxidation number to receive full credit a. Ca(s) + LiCl(aq) 3 QUESTION III B. 1st: Predict and balance the molecular equation. 2nd: Write the ionic and total net ionic equation. If no reaction takes place, then write NR for the total net ionic equation. 1. ……..Mg(NO3)2(aq) + ……Na2S(aq) 2. ………K3PO4(aq) + …….Ca(NO3)2(aq) 3. ……….HCN(aq) + ……..Ca(OH)2(aq) 4 Question IV A. A useful application of oxalic acid is the removal of rust (Fe2O3) from, say, bathtub rings according to the reaction. Fe2O3(s) + 6 H2C2O4(aq) 2Fe(C2O4)3 (aq) + 3H2O + 6H+(aq) Calculate the number grams of rust that can be removed by 5.00 x 102 mL of a 0.100 M solution of oxalic acid. Remember to set up your problem with stoichiometry 1.32 g B. Calculate the density (g/L) of hydrogen bromide (HBr) gas in grams per liter at STP. Assume the volume of solution is 1.00L 3.61 g/L 5 Question V In the laboratory, hydrogen gas is usually made by the following reaction: Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq) How many liters of H2 gas, collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C, can be made from 1.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C 0.599L B. A balloon contains 0.76 mol N2, 0.18 mol O2, 0.031 mol He, and 0.026 mol H2 at 739 mm Hg. i. What is the partial pressure of He? 23 mmHg or 0.030 atm ii. What is the mole fraction of N2? 0.76 6











