Unit 5: Acids and Bases
advertisement

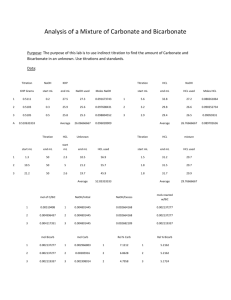
5.3.1 Neutralization reactions 5.3.2 Titration Reactions What happens when an acid such as HCl is mixed with a base such as NaOH: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) When an acid and a base are combined, water and a salt are the products. Salts are ionic compounds containing a positive ion other than H+ and a negative ion other than the hydroxide ion, OH-. Double displacement reactions of this type are called neutralization reactions. We can write an expanded version of this equation, with aqueous substances written in their longer form: H+(aq) + Cl-(aq) + Na+(aq) + OH-(aq) → Na+(aq) + Cl(aq) + H2O(l) Removing the spectator ions we get the net ionic equation: H+(aq) + OH-(aq) → H2O(l) When a strong acid and a strong base are combined in the proper amounts ([H+] equals [OH-]) - a neutral solution results (pH = 7). But, salt solutions do not always have a pH of 7. Through a process known as hydrolysis, the ions produced may react with water molecules to produce a solution that is slightly acidic or basic. Generally if a strong acid is mixed with a weak base there the resulting solution will be slightly acidic; if a strong base is mixed with a weak acid the solution will be slightly basic. Acid-base titrations are lab procedures used to determine the concentration of a solution During an acid-base titration, an acid with a known concentration (a standard solution) is slowly added to a base with an unknown concentration (or vice versa). A few drops of indicator solution are added to the base. The indicator will change colour, when the base has been neutralized (when [H+] = [OH-]). At that point - called the equivalence point or end point - the titration is stopped. By knowing the volumes of acid and base used, and the concentration of the standard solution, calculations allow us to determine the concentration of the other solution. It is important to accurately measure volumes when doing titrations. The instrument you would use is called a burette (or buret). 1.Rinse the burettes several times with the acid and base solutions, then fill them with the appropriate solution. 2.Using the burette, accurately measure a volume of the base into an Erlenmeyer flask. How much you use is not important, as long as you know exactly how much is in the flask. 3.Add a suitable indicator such as 4. The acid (with the known concentration - the titrant) is then added to the base. Release the acid from the burette quickly at first, then more slowly as the endpoint is neared. How do you know when you are reaching the endpoint? The indicator will begin to show a change in colour. Swirling the flask will cause the colour to disappear. ENDPOINT IS REACHED AS SOON AS THE COLOUR CHANGE IS PERMANENT. ONE DROP WILL DO IT - once the colour change has occurred, stop adding acid. If a pH meter is used instead of an indicator, endpoint will be reached when there is a sudden change in pH. 5. Record the volume of acid added to reach endpoint. Calculations will allow us to calculate the concentration of the base. 6. The titration should be repeated several times and the results averaged. Often the first trial is done quickly to get a general idea of the volume of titrant required. Subsequent trials are done more slowly. Use titration information to calculate the concentration of the unknown solution, you must know the following information. The concentration of one of the solutions, the acid for example (MA) The volume of acid used for the titration (VA) The volume of base used for the titration (VB) What you will calculate: The concentration of the other solution, the base for example (MB) MAVA = MBVB During a titration 75.8 mL of a 0.100 standard solution of HCl is titrated to end point with 100.0 mL of a NaOH solution with an unknown concentration. What is the concentration of the NaOH solution? MA= 0.100 M MB = MB VA =75.8 mL VB =100.0 mL Substitute in known values and solve for the unknown: MAVA = MBVB (0.100)(75.8) = MB(100.0) 7.58 /100.0 = MB 0.0758 = MB Answer: The concentration of the NaOH solution is 0.0758 M or 7.58 ×10-2M A 20.0 mL solution of strontium hydroxide, Sr(OH)2, is placed in a flask and a drop of indicator is added. The solution turns colour after 25.0 mL of a standard 0.0500M HCl solution is added. What was the original concentration of the Sr(OH)2 solution? Write a balanced equation for the neutralization reaction: 2 HCl(aq) + Sr(OH)2 (aq) → SrCl2 (aq) + H2O(l) Notice that 2 moles of the acid HCl are required to neutralize 1 mole of the base Sr(OH)2. Define the variables to be used and the known values: MA = 0.050 M MB = MB VA = 25.0 mL VB = 20.0 mL To balance this out we need to modify our formula: MAVA = 2 MBVB Notice that the "2" is on the base side of our formula even though it was in front of the acid side in the balanced equation - it "switches places". Substitute in known values for the equation and solve for the unknown: MAVA = 2MBVB (0.050)(25.0) = 2MB(20.0) 1.25 = 40.0MB 1.25/ 40.0 = MB 0.0312 = MB Answer: The concentration of the Sr(OH)2,solution is 3.12 ×10-2M. (You will not be required to know this section for a test.) What indicator is used depends on what type of titration you are performing. An indicator should be chosen that will change color when enough of one substance (acid or base) has been added to exactly use up the other substance. The three main types of acid-base titrations, and suggested indicators, are: Titration between . . . Indicator 1. strong acid and strong base any 2. strong acid and weak base methyl orange 3. weak acid Explanation phenolphthalein changes color in the acidic range(3.2-4.4) changes color and strong base in the basic range (8.2 - 10.6) Lab 5.3.2- titrations Assignment 5.3.2