CIS- Chemistry - Mountain View Los Altos District
advertisement

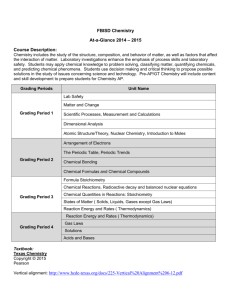
MVHS COURSE INFORMATION SHEET Course Title: Chemistry Instructor: Dr. Thornburg UC/CSU: Yes/Yes Textbook and other learning resource: : Holt Chemistry, CA Ed., Myers, Oldham, & Tocci What Students Can Expect From This Course Course Description: Chemistry is the branch of science that deals with the composition of matter and the changes in composition that matter can undergo. In investigating the composition of matter, we will explore the structure of the atom and the ways in which that structure determines the kinds of substances that can be formed. In studying changes in composition, we will integrate this structure with the energy considerations that govern chemical reactions. We will see how the science has developed and how its models have been tested and shown to have predictive power. Our exploration will take us from the fundamental ideas associated with chemical and physical change, to an understanding of the interactions of atoms, ions, and molecules, quantifying their reactions, and predicting reaction products. As well as learning about the unique properties of gases, we will address equilibrium situations and the rates of reactions, and conclude our studies with an in depth treatment of aqueous chemistry. to understand behavior of gases, the conditions of for the properties of liquids and solids. We consider the electronic structure inside the atom, chemical equilibrium and reaction rates. Then we elaborate our theoretical ideas in an extended treatment of aqueous chemistry. Finally we apply the principles we have learned to the realm of organic chemistry as we examine a few of the basic building blocks of life. This course is designed to provide you with knowledge of the fundamental concepts called chemistry. It will also reinforce your understanding of the chemical basis of the biology you encountered last year. We will focus on strengthening your logical reasoning processes, enhancing your problem solving strategies, and developing your skill in organizing the products of scientific thought and experimentation into coherent reports. As you continue to grow in your ability to use the scientific method in approaching problems, you will become familiar with a wide variety of laboratory techniques. Thus, your expected learning outcomes lie in the development of: Critical thinking skills - as you employ the scientific method of reasoning to proceed logically from observations to inferences and conclusions based on those inferences. You will draw on your analytical abilities when you connect experimental error to the source of that error in your execution of experiments. You will apply your knowledge of relevant chemical concepts as you solve problem sets that are designed to expand your knowledge of the physical implications of those concepts. On the completion of each unit you will reflect on the key ideas embodied in that unit, and be able to highlight those ideas in written form. Problem-solving skills - as you identify the given information and apply relations that connect the pieces of a scientific problem to produce logical and testable solutions both on paper and in the lab. Specifically, you will learn to extract meaningful information from written problem situations using a reciprocal teaching model. Even more important, you will become able to use the technique of dimensional analysis to solve problems in a logically consistent format. Communication skills - as you discuss concepts and problem solve together, and as you present the results of scientific investigations in lab notebook writeups. In addition, you will be presenting solved problems to the class as a whole, and will field questions from your classmates as they attend to your solution process. Learning acquisition skills - as you interact with the scientific concepts, hypothesize and test your hypotheses and come fully to understand that learning chemistry is not a spectator sport. Learning science involves a constant back and forth interaction between the source of concept learning (class notes and text) and the application of those concepts to the solution of the problems in your problem sets. The problems in your problem sets were selected to promote this interaction. You will also be developing your ability to use this technique of learning science when you analyze your data and results from your laboratory experiments. Creativity - as you build mental models of the behavior of atoms and molecules.. You will have the opportunity to illustrate concepts associated with the electronic structure of atoms and molecules and periodic table trends via writing fanciful stories and develop thematic posters that anthropomorphize aspects of these entities. Computer skills - as you develop and present projects using word processing and graphing programs and work with chemistry simulation programs. In particular, we will be using Exploring Chemistry software in group discussion formats to give meaning to equilibrium notions and solution reactions. Integrated reading/math/writing skills - as you master logical arguments in text form, apply mathematical operations in the expression of scientific laws and transmit the results of those activities in written form. An appreciation for the role of science and technology in modern society - as you explore applications of chemical principles to the development of materials, technologies, and to the preservation of our environment. You will be reading many articles that link chemistry to real world situations. These articles appear in Chemistry Matters, a magazine written especially for high school students. Mountain View High School Mission To increase the instructional coherence within and between departments while raising the achievement of all students Assessment and Grading: Per BP/AR 5121, teachers of the same course are expected to align their grading and assessment practices in the following areas: (a) the weight of assignments for the various categories of assessment, (b) homework policies, late/make up policies, opportunities for revision, extra credit and grading scales, (c) degrees of proficiency. In addition, the policy specifies that “group work is to be considered an essential part of the learning experience, and that grades earned through group participation are to reflect an individual student’s achievement on a designated academic standard and to be awarded to individuals rather than to groups of students. Extra credit is to be given only when it supports student achievement of academic standards for the course and when it is equitable for all students. Grading practices are determined by course teams. All teachers of this course will determine grades as defined below: 1. 2. 3. 4. 5. 6. 7. 8. Weight of assignments and/or components of the grade Grading Scale Homework policy Late work, missing work and opportunities for revision Group work Extra credit How proficiency is determined/how student work is assessed Grade Book Update Policy Students are expected to master the details of the following concepts and skills· Concepts The chemical elements are fundamental building materials of matter. All matter can be understood in terms of arrangements of atoms. Atoms retain their identity in chemical reactions. Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them. Skills The student can use representations and models to explain scientific observations and solve scientific problems. The student can use dimensional analysis in problem solving. Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons. The student exercises scientific reasoning when planning and carrying out experiments. The student can connect the outcome of an experiment to the methodology of the experiment. Rates of chemical reactions are determined by the details of molecular collisions. The student can work with scientific explanations and theories. Energy plays an essential role in the changes matter undergoes. The laws that describe this role can be used to predict what changes will occur. The student can identify relevant information in chemical problems and can connect that information appropriately in arriving at problem solutions. Although grades are determined on a percentage of "total points" basis, assignment point values are scaled so that Summative Test scores determine roughly SO% of your grade, Lab Reports amount to about 38% of the grade, Miscellaneous Assignments and In-Class Work are worth 6-8%, and a Work Habits grade is 4-6%. Work habits are assessed by the number of daily homework stamps you accumulate and by your cooperation in making the class go well. The “total points” grade ranges are 80-100% A – Exemplary, 60-79% B – Proficient, 40-59% C – Progressing, 20-39% D – Basic, 0-19% F- Below Basic. Proficient on Tests means: The student has demonstrated an above average ability to extract relevant information from the questions to solve the problems effectively. The student can select and apply relevant standards-based concepts in solving chemical problems. The student has an adequate understanding of the use of chemical language and mathematical conversion in representing concepts symbolically and in manipulating those concepts mathematically. The student has a limited understanding of the scope of the standard and thus can occasionally d draw together some related concepts and integrate them correctly in problem solving. Proficient in writing Lab Reports means: The student has demonstrated an above average grasp of the purpose and outcome of the experiment. The report proceeds logically from the data obtained through the means of obtaining it to the results obtained and the conclusions that can legitimately be drawn from those results. Calculations may be incomplete or results not fully discussed. Minor omissions with respect to units or equipment specification may exist. Error possibilities are too general and not clearly linked to the outcome or not supported by procedural and observational details. A separate rubric defines the remaining levels of mastery for Tests and Lab Reports. Multiple opportunities for demonstrating your level of mastery are offered. Extra Credit, however is not offered, with the exception of Mole Day. Mole Day Extra Credit will appear in the Work Habits category. Any bond or intermolecular attraction that can be formed can be broken. Reaction conditions can influence which of these two competing processes will prevail. Mountain View High School Mission To increase the instructional coherence within and between departments while raising the achievement of all students What Is Expected Of The Student Daily Assignments: Please purchase a laboratory notebook and a scientific calculator. You will need to have a three-ring binder with dividers for your notes and workbook sheet storage. Colored pencils are an optional asset. The notes you take in class are invaluable in helping you to master the material of this course. You will need to spend 10 - 15 minutes each night learning these class notes, as well as an additional 15 - 20 minutes working problems, writing up labs, reading the referenced text sections, and/or completing related homework. Each day’s lesson builds on the preceding day’s work. Do not let yourself get behind. You will be asked to apply the information presented to solve chemical problems. This process requires you first familiarize yourself with the concepts and techniques presented in class. As you attempt the problems you may find you need to go back and reread your notes or text and rethink through the ideas that have been presented. This is normal. Because of the sequential and cumulative nature of teaching and learning chemistry, dally assignments that constitute make-up work (arising from absences) will generally be stamped. In the case of unexcused absence, no stamp will be issued. There is no leeway granted on stamping dally work that is not done. A few days leeway Is customarily granted on other work provided you are seeking help from me or In the Tutorial Center to enable you to complete the given assignment. Attendance: ABSENCES: An “unexcused absence” is an absence in excess of a 30-minute period occurring in a given class. Students may not exceed 14 unexcused absences across their entire schedule. A full day, unexcused absence counts as 5, 6 or 7 absences depending on how many classes a student carries toward the total of 14. On the 15th unexcused absence, students may be referred to an alternative educational program/site pursuant to the District’s involuntary transfer policy (AR/OP 5113). TARDIES: Students may not exceed 19 unexcused tardies across their entire schedule. An unexcused tardy is an absence from class from when the bell rings until up to 30 minutes of a class period. At the 15th unexcused tardy, there will be a mandatory parent conference with the student’s Assistant Principal. This conference will be scheduled to occur the morning after the family is contacted by the school. At the student/parent conference, consequences for continued tardiness are discussed and the student is assigned to Saturday School. An attendance contract will also be signed at this meeting. Failure to attend Saturday School may result in a transfer to an alternative educational program/site. Classroom Rules: We operate In an atmosphere of mutual respect and consideration. That respect extends to the chemicals and the equipment you will be using. I will not allow anyone to work in the laboratory who Is not wearing SAFETY GOGGLES/FACE MASK or who is not following safe operating procedures. There is no eating or drinking In the lab area. Students are invited to review past tests during their free periods and at lunch. However, no problems may be copied, and under no circumstances may a test be removed from the Science building unless done so under the auspices of a Special Education Teacher or Special Education Aide. Scratch work should be turned In to me before leaving the building. Students are encouraged to identify the concepts that have given them trouble, and to practice these concepts by locating workbook problems that involve these concepts in arriving at solutions. Violation of this policy will be deemed as cheating and iIs subject to school disciplinary action. Cheating Policy: The Board expects that students will not cheat, lie, plagiarize or commit other acts of academic dishonesty. Examples of cheating include: anyone who copies another’s work or turns in someone else’s ideas as his or her own, collaboration with another student or students could be considered cheating if students are expected to complete an assignment independently, copying homework, allowing someone else to copy your work, plagiarism, copying or allowing others to copy from another’s exam, improperly obtaining and/or using tests, questions, or answer keys, using unauthorized notes/materials or electronic equipment (calculators, cell phones, etc.), with greater access to the Internet and electronic sources, students need to be very clear about their responsibilities in using these tools with integrity. Check with your teachers if you are unsure or unclear about his/her expectations regarding the use of the Internet. Help and Contact Information Phone: (650) 940-2462 Email: Katie.Thornburg@ mvla.net Please contact me by email if at all possible. Mountain View High School Mission To increase the instructional coherence within and between departments while raising the achievement of all students