Chapter 5 - OSU Chemistry
advertisement
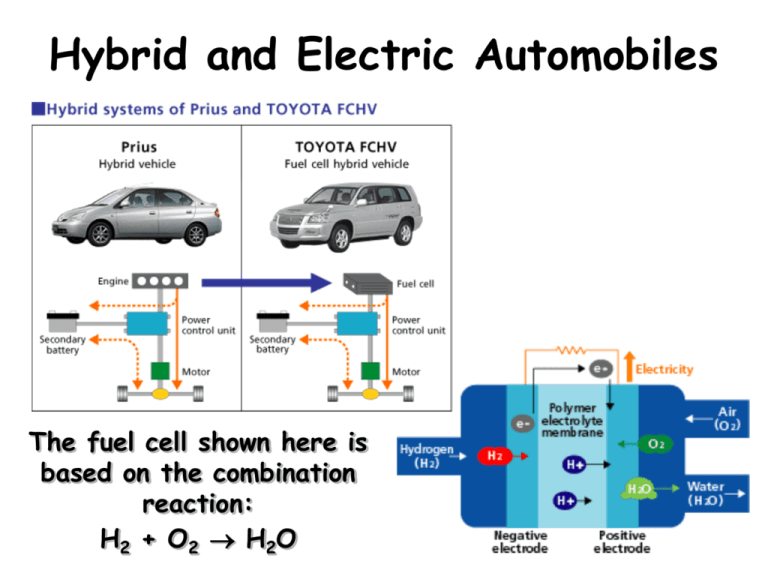
Hybrid and Electric Automobiles The fuel cell shown here is based on the combination reaction: H2 + O2 H2O Definitions Thermodynamics The study of energy and it’s transformations. Thermochemistry The thermodynamics of chemical reactions. Energy The capacity to do work or transfer heat. Work The energy required to move an object against an opposing force. W = F d Heat Derived from the movements of atoms and molecules (including vibrations and rotations). Types of Energy Kinetic Energy The energy of motion, energy in action. Ek = (1/2)mv2 Potential Energy The energy of position, stored energy. Work & Chemical Reactions For the vast majority of chemical reactions there are two types of work that can happen. – Mechanical Work – done by creating/destroying a gas (i.e. automobile cylinder, air bag, etc.) – Electrical Work – A redox reaction flowing through an external circuit (i.e. battery, fuel cell) 1st Law of Thermodynamics Energy is neither created nor destroyed (conservation of energy) Internal Energy = heat + work DE = q + w Enthalpy Enthalpy (H) The change in enthalpy, DH, is defined as the heat gained or lost by the system under constant pressure. DH = qp Where qp = the heat flow at constant pressure Properties of Enthalpy 1. Enthalpy is a state function. 2. Enthalpy is an extensive property. 3. Enthalpy is reversible. If we reverse the reaction the magnitude of DH remains the same but the sign changes. 4. The enthalpy change depends upon the states of the reactants and products. Constant-Volume (Bomb) Calorimetry Commonly used for studying reactions, especially combustion reactions. Because pressure isn’t constant, the bomb calorimeter measures DE rather than DH Constant-Pressure Calorimetry We’ll use this kind of calorimeter in lab 1. Calibrate the calorimeter in order to determine its specific heat. 2. The calorimeter minimizes heat transfer into or out of the system. 3. By measuring the change in the temperature of the solution, we can calculate the heat given off in a reaction. Energy Stored in Carbs/Sugar The body breaks carbohydrates down into glucose (blood sugar), C6H12O6, which is then combusted in our bodies C6H12O6 (s) + 6O2(g) 6CO2(g) + 6H2O(l) DH° = -2803 kJ In terms of energy released per gram of glucose one mole of glucose has a mass of 180 g so that -2803 kJ/(180 g/mol) = 16 kJ/g Fuel values for foods are typically reported in Calories, which is stands for Kilocalories or kcal 1 Cal = 1000 cal 1 cal = 4.184 J 1 kcal = 4.184 kJ So for glucose 16 kJ/g x (1 kcal/4.18 kJ) = 3.7 kcal/g Energy Stored in Fats If we take a typical fat, such as tristearin, C57H110O6 the reaction with oxygen in our cells is: 2C57H110O6 (s) + 163 O2(g) 114CO2(g) + 110H2O(l) DH° = -75,520 kJ In terms of energy released per gram one mole of tristearin has a mass of 891 g so that -75,520 kJ/(2891 g/mol) = 42 kJ/g In terms of kcal (Calories) tristearin would be 42 kJ/g x (1 kcal/4.18 kJ) = 10 kcal/g In general carbohydrates and proteins have an average fuel value of 4 kcal/g and fats are 9 kcal/g. This is what you see as Calories on food labels. Energy Stored in Fuels Octane (2,3,5 trimethyl pentane, DHº = -255 kJ/mol) 2 C8H18 (l) + 25 O2(g) 16 CO2(g) + 18 H2O(g) DH° = -10,138 kJ -10,138 kJ/(2114 g/mol) = -44 kJ/g Ethanol (DHº = -278 kJ/mol) C2H5OH (l) + 3 O2(g) 2 CO2(g) + 3 H2O(g) DH° = -1234 kJ -1234 kJ/(31 g/mol) = -40 kJ/g Methane (DHº = -75 kJ/mol) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) DH° = -802 kJ -802 kJ/(16 g/mol) = -50 kJ/g Hydrogen (DHº = 0 kJ/mol) 2 H2 (g) + O2(g) 2 H2O(g) DH° = -484 kJ -484 kJ/(22 g/mol) = -121 kJ/g