K i - Tesionline
advertisement

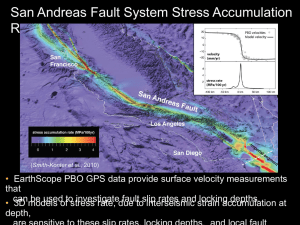
No. 52f4f0750705145539 Second generation PBOs show enantioselective binding to the catalytic complex of HIV-1 RT wt and drug resistant mutants. Alessandra Amoroso*, Alberta Samuele*, Aristotele Karytinos*, Marcella Facchini*, Samantha Zanoli*, and Giuseppe Campiani°, Giovanni Maga* *DNA Enzymology and Molecular Virology, Institute of Molecular Genetics IGM-CNR, National Research Council, 27100 Pavia, Italy; °Department of Pharmaceutical Chemical Technology and European Research Center for Drug Discovery and Development, University of Siena, 53100 Siena, Italy. INTRODUCTION The virus-encoded human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) is a major target for antiviral chemotherapy. Two classes of inhibitors are targeted to the viral RT: nucleoside RT inhibitors (NRTIs) and nonnucleoside RT inhibitors (NNRTIs). The latter compounds are highly specific for HIV-1 RT and bind to the enzyme at an allosteric site close to, but distinct from the catalytic site, behaving as typical non competitive inhibitors with respect to the different substrates of the polymerization reaction. The occurrence of just a few single amino acid substitutions in the non-nucleoside binding site (NNBS) of the RT may confer resistance to most of the NNRTIs. Therefore, the development of novel NNRTIs with improved pharmacological, pharmacokinetic, and drug resistance mutation profiles, is critical for a more successful application of NNRTIs in combination therapies with other anti-HIV drugs. In this study we investigated some analogs of pyrrolobenzoxazepinone derivatives (PBOs), previously described as effective non nucleoside inhibitors (NNRTIs) of HIV-1 RT, and their corresponding R and S enantiomers, in order to evaluate their interactions with RT wild type and the drug-resistant mutants K103N, L100I and V179D. MATERIALS AND MEHODS The HIV-1 RT gene fragment spanning codons 2 to 261 from pHXB2 2-261RT constructs carrying K103N, L100I and V179D mutations was amplified by PCR, digested with Acc1 and Pvu2 and cloned into the expression plasmid p6HRT (∆Xho1/Bgl2) containing the wild type RT gene. The resulting p6HRT expression vectors with K103N, L100I and V179D mutations were used for the production in E. coli (BL21) and purification of recombinant His-tagged RT enzymes in a Fast Protein Liquid Chromatography (F-PLC) system, using Ni-NTA superflow column (QIAGEN, U.S.A.). All the enzymes were purified to a >95% purity, as confirmed by Coomassie stain, and had a specific activity on poly(rA):oligo(dT). RNA-dependent DNA polymerase activity of HIV-1 RT, either wt or mutants, was assayed using a working reaction buffer (TDB 1X) containing 50mM Tris-HCl (pH 8.0), 1mM dithiothreitol (DTT), 0,2 mg/ml bovine serum albumine (BSA), 2% glycerol. Thus, 2-4 nM RT were incubated at 37°C with 10 mM MgCl2, 0,5 mg of poly(rA):oligo(dT)10:1 (0,3 mM 3’–OH ends) , 10 mM radioactive 2’deoxy-thymidine 5’-triphosphate (3[H]dTTP) (1 Ci/mmol), for the indicated time. Then, 20 ml aliquots were spiked on glass fiber filters GF/C (Whatman Int. Ltd, Maidstone, England) and, immediately, immersed in 5% ice-cold trichloroacetic acid (TCA) (AppliChem GmbH, Darmstadt). Filters were washed three times with 5% TCA and once with ethanol for 5 minutes, then dryed and, finally, added with EcoLume® Scintillation cocktail (ICN, Research Products Division, Costa Mesa, CA USA), to detect the acid-precipitable radioactivity. Incorporation of radioactive dTTP into poly(rA):oligo (dT) was quantitated by testing increasing amounts of inhibitors [0,01-40 mM]. All other chemicals were of analytical grade and provided by Sigma-Aldrich (St. Louis, MO, USA), or Merck and Boehringer. Steady state kinetic assays were performed to assess the equilibrium association and dissociation constants of the different enzyme-inhibitor complexes as indicators of the affinity of PBOs for the catalytic forms of HIV-1 RT, either wt or mutated. The inhibition of HIV-1 RT exerted by the different PBO compounds was analyzed as a function of both the nucleic acid and the nucleotide substrates, varying the concentrations of one of the substrates while the other was maintained constant and saturating. The time-dependent incorporation of 3[H]dTTP into poly(rA):oligo(dT) at different substrate concentrations was monitored by removing 25 µl-aliquots at different time points. Data obtained were analyzed by non linear regression analysis using 3.0 GraphPad Software (San Diego, CA, USA). All values were calculated by non-least-squares computer fitting of the experimental data to the appropriate rate equations. RESULTS (I) We first evaluated the HIV-1 RT RNA-dependent DNA polymerase activity with increasing concentrations of selected PBOs and their corresponding R,S enantiomers against either RT wt or mutants (Fig. 1A-D) Most of the S enantiomers exhibited modest or no inhibition against both RT wt and mutants. On the other hand, the R isomers PBO 438 R, 1027 R, 1060 R inhibited all the enzymes with activities comparable to those of the corresponding racemic mixture, indicating a stereoselective mode of action for these derivatives. Conversely, PBO 1108 showed a modest inhibitory effect towards RT wt, while no activity was appreciable against any of the mutants, whereas PBO 1028 R showed some activity towards RT wt and the K103N mutant, but was inactive towards the L100I and V179D enzymes. Steady-state kinetic analysis of the interaction of HIV-1 RT with varying concentrations of poly(rA):oligo(dT) and dTTP substrates, in presence of fixed amounts of PBOs, has been performed to determine the kinetic parameters VMAX, Km and kcat. Our results confirmed that all these PBO derivatives acted by a fully non-competitive mechanism, since they did not significantly influence the affinity of the enzyme for its substrates, affecting the sole rate of the reaction. RESULTS (II) All wt and mutated RTs were tested on poly(rA):oligo(dT) in the presence of the three different PBOs with varying concentrations of dTTP. The apparent inhibition constant Ki app values for the different inhibitors at each substrate concentration were calculated. Its variation as a function of dTTP was analyzed, to calculate the true equilibrium dissociation constant for the ternary complex Kiter. All the three inhibitors assayed showed an increase of potency, indicated by the gradual reduction of the Ki app values, as dTTP concentrations were increased, indicating a selective binding of the compounds to the ternary complex, in agreement with previous studies. The Kiter values, evaluated for PBO 438-R, 1027-R and 1060-R, were compared with the corresponding affinities for the free enzyme (Kifree) and the binary complex (Kibin). Kiter values against RT wt were approximately decreased 4-fold for PBO 438 R and 1027 R, and 9-fold for PBO 1060 R with respect to the Kifree/bin. Analogously, in the case of K103N, Kiter values were reduced approximately 2-fold for PBO 438-R and 1027 R, whereas a 25-fold reduction was observed for PBO 1060 R. Concerning the mutant L100I, Kiter values were decreased 1.3-fold for PBO 438-R and 1060 R, while a 40-fold reduction was observed for PBO 1027 R. Finally, in the case of the V179D mutant, we observed a 1.4-fold reduction in Kiter levels for PBO 438-R, 3.3-fold for 1027 R, and 1.2-fold for PBO 1060 R. RESULTS (III) These different compounds seemed to have selective specificities for different enzymes. This is shown as relative resistance indexes for the various inhibitors. The compound 1060 R, beside showing the highest potency towards all the mutants tested, proved to be the most selective in targeting the ternary complex of K103N, whereas the compound PBO 1027 R showed the highest selectivity towards L100I. As a result, PBO 1027 R was active towards L100I more than towards the wt, whereas PBO 1060 R inhibition against K103N was quite comparable to that exhibited against wt. In order to determine the basis for the observed resistance, we compared the association (kon) and dissociation (koff) rates of the PBOs for all the enzymes. The compound PBO 438 R showed a decrease in the association rates (kon) between 5- ad 10-fold towards the ternary complexes of all the mutants tested, whereas no significant differences were noted for the corresponding dissociation rates (koff). The compound PBO 1027 R also showed a relevant reduction in the kon values, which was compensated by a concomitant reduction in its dissociation rates. The compound PBO 1060 R, on the other hand, showed a simultaneous reduction of the kon and koff rates for the K103N mutant, whereas to a modest decrease in the kon rates for the mutants L100I and V179D corresponded a significant increase of the koff rates for these enzymes. DISCUSSION AND CONCLUSIONS NNRTIs have a significant role in combinatorial anti HIV therapies. Because of their importance and in order to bypass the high incidence of resistance mutations, research has focused on developing a large pool of effective drugs, starting from well-known molecules. In this study we evaluated a group of compounds from the second generation PBOs, in which the aromatic side chain is extended in order to improve the interaction between the molecule and the primer-grip region of the Non Nucleosidic Binding Site (NNBS), in particular with the W229 residue. Since a favorite position for PBOs in the NNBS has not been identified yet, in the present work the behavior of R and S enantiomers was observed for each compound tested. Activity studies on wt and mutant enzymes allowed us to select three compounds for further studies. The results of the inhibition assays showed that, while R enantiomers significantly reduced enzymatic activity, S enantiomers scarcely affected the reaction. The data obtained from the study of the kinetic parameters on the selected compounds confirmed a non competitive mode of inhibition, suggested by a reduction of the kcat values at increasing concentrations of inhibitors, while the Km values remained constant. In addition, comparisons between Ki free-bin and Ki ter values indicated an increased affinity of tested compounds for the catalitically competent ternary complex respect to the free enzyme and the binary complex, in accordance to the ordered mechanism proposed for RT. Deepening our knowledge on the molecular basis of the interaction, we also evaluated the association (kon) and dissociation (koff) rates, in order to determine if any reduction of the inhibitory activity of the mutants respect to that of wt came from a reduction of the association rate or an increase of the dissociation rate. Taken together, these data also indicated different selectivities of the compounds tested for the mutant forms of RT. Our results suggested a possible use of these compounds into an anti-HIV therapy; it is necessary, however, to conduce further toxicity and farmacokinetic studies. The data collected in the present work expand our knowledge on NNRTIs, providing important information for development of wide-range antiviral drugs. No. 5793e5140705122433 Characterization of novel N,N-DABOs as effective tight binding non-nucleoside inhibitors of HIV-1 RT Marcella Facchini*, Alberta Samuele*, Lidia Ferrigno*, Aristotele Karytinos*, Dante Rotili°, Antonello Mai°, Giovanni Maga* *DNA Enzymology and Molecular Virology, Institute of Molecular Genetics IGM-CNR, National Research Council, 27100 Pavia, Italy; °Pasteur Institute, Cenci-Bolognetti Foundation, Department of Pharmaceutical Research, University of Study “La Sapienza” Rome. INTRODUCTION Reverse transcriptase (RT) is a key multifunctional enzyme in the life cycle of the human immunodeficiency virus type-1 (HIV-1), the causative agent of AIDS, and represents a major target for antiviral chemotherapy. RT inhibitors are usually classifiable in two main groups: nucleoside inhibitors (NRTIs) and non-nucleoside inhibitors (NNRTIs). The NRTIs interact competitively with the catalytic site of the RT, whereas NNRTIs interact with an allosteric site adjacent to the catalytic site of RT. The occurrence of just a few single amino acid substitutions in the non-nucleoside binding site (NNBS) of the RT may confer resistance to most of the NNRTIs. Therefore, the development of novel NNRTIs with improved pharmacological, pharmacokinetic, and drug resistance mutation profiles, is critical for a more successful application of NNRTIs in combination therapies with other anti-HIV drugs. In this study we investigated some 2-chloro-6-fluoro analogs of N,N-dihydro-alkoxy-benzyl-oxopyrimidine derivatives (N,N-DABOs), previously described as effective HIV-1 NNRTIs, characterized by a broad activity spectrum against NNRTIs resistant mutants. We performed on these compounds binding mode and kinetic analyses, comparing results obtained with that of 2,6 F2-substituted, previously described as slow-binding tight-binding inhibitors. MATERIALS AND METHODS Condensation of N,N-dimethylguanidine hydrogensulfate with the appropriate b-oxoesters in the presence of sodium ethoxide in ethanol afforded the compounds MC1838, MC1839, MC1778 and MC1784 in high yields. Compounds MC1236 and MC1281 were obtained by nucleophilic displacement of the C2 methylthio group of the 5-methyl-2-methythio-6-[1-(2,6-difluorophenyl)ethyl]-3,4-dihydropyrimidin-4(3H)one with piperidine or thiomorpholine respectively. The HIV-1 RT gene fragment spanning codons 2 to 261 from pHXB2D2-261RT constructs carrying K103N and Y181I mutations was amplified by PCR, digested with Acc1 and Pvu2 and cloned into the expression plasmid p6HRT (∆Xho1/Bgl2) containing the wild type RT gene. The resulting p6HRT expression vectors with K103N and Y181I mutations were used for the production in E. coli (BL21) and purification of recombinant His-tagged RT enzymes in a Fast Protein Liquid Chromatography (F-PLC) system, using Ni-NTA superflow column (QIAGEN, U.S.A.). All the enzymes were purified to a >95% purity, as confirmed by Coomassie stain, and had a specific activity on poly(rA):oligo(dT). RNA-dependent DNA polymerase activity of HIV-1 RT was assayed incubating 2-4 nM RT at 37°C in a working reaction buffer (TDB 1X) containing 50mM Tris-HCl (pH 7.5), 1mM dithiothreitol (DTT), 0.2 mg/ml bovine serum albumine (BSA) and 0.2% glycerol with 10 mM MgCl2, 0.25 mg of poly(rA):oligo(dT)10:1 (0.2 mM 3’-OH ends) and 10 mM radioactive 2’deoxy-thymidine 5’-triphosphate (3[H]dTTP) (1 Ci/mmol), for the indicated time. Then, 20 ml aliquots were spiked on glass fiber filters GF/C (Whatman Int. Ltd, Maidstone, England) and, immediately, immersed in 5% ice-cold trichloroacetic acid (TCA) (AppliChem GmbH, Darmstadt). Filters were washed three times with 5% TCA and once with ethanol for 5 minutes, then dried and, finally, added with EcoLume® Scintillation cocktail (ICN, Research Products Division, Costa Mesa, CA USA), to detect the acid-precipitable radioactivity. All other chemicals were of analytical grade and provided by Sigma-Aldrich (St. Louis, MO, USA), or Merck and Boehringer. In order to evaluate the potency of the inhibitors (Ki), incorporation of radioactive dTTP into poly(rA):oligo(dT) was quantitated in presence of increasing amounts of inhibitors [0.2-5 Ki], upon preincubation with the enzyme and subsequent addition at different time points ( 2’, 5’, 10’, 20’) of the working reaction mixture. Steady state kinetic assays were performed to assess the equilibrium association (kon) and dissociation (koff) rates of the different enzyme-inhibitor complexes as indicators of the affinity of DABOs for the three catalytic forms of HIV-1 RT, either wt or mutated. The time-dependent incorporation of 3[H]dTTP into poly(rA):oligo(dT) at different substrate concentrations was monitored by removing 50 µl-aliquots at different time points. Data obtained were analyzed by non linear regression analysis using 3.0 GraphPad Software (San Diego, CA, USA). All values were calculated by non-least-squares computer fitting of the experimental data to the appropriate rate equations. Ki (mM) K103N MC 1236 0.070 0.600 ± 0.01 ± 0.05 MC 1281 0.060 10.000 ± 0.01 ± 1.12 MC 1838 MC 1839 Y181I Relative Resistance Index (RRI) RESULTS (I) Ki m ut / Ki w t n.a. 312.5 350 300 n.a. 0.003 1.000 6.042 ± 0.0002 ± 0.09 ± 0.59 0.017 0.750 0.424 ± 0.001 ± 0.08 ± 0.04 -fold resistance RT wt 250 K103N 166.7 200 Y181I 150 100 50 42.9 8.6 48.9 24.3 19.7 0 MC 1778 0.712 14.000 ± 0.06 ± 1.54 MC 1784 0.307 15.000 ± 0.03 ± 1.63 n.a. -50 MC1236 MC1281 n.a. MC1838 MC1839 MC1778 MC1784 We first evaluated the HIV-1 RT RNA-dependent DNA polymerase activity with increasing concentrations of selected DABOs against either RT wt or mutants. Ki values, so obtained, indicated that our compounds were active at nanomolar concentrations against RT wt, especially MC1838 (see table). In order to assess the drug-resistance effects of the mutations K103N and Y181I, we also calculated the relative resistance index (RRI) for each compound, although most of them showed no significant activity against Y181I, with the exception of MC1839. All the inhibitors showed RRI >1 (Fig. 1), this confirming their lower affinity towards the mutants compared to wt. The compound MC1236 proved most effective towards K103N, while MC1838 unexpectedly presented the greatest RRI, due to the low entity of its Ki value on wt, affecting the ratio. Figure 1: Relative Resistance Index (RRI) Relative Association Index (RAI) kon wt / kon mut Association Rates (kon ) 1.0000 RESULTS (II) 2341.9 0.703 0.8000 2500 0.4918 0.6000 RT wt 0.4000 0.2000 -fold association kon ( s-1 m M-1) We performed binding and kinetic assays in order to obtain the association (kon) and dissociation (koff) rates for the three structurally distinct mechanistic forms of RT: the free enzyme (E), the binary complex of RT with the template-primer (E:NA), and the catalytically competent ternary complex of RT with both nucleic acid and nucleotide (E:NA:dNTP). We observed that 2,6-Cl,Fderivatives (MC1838 and MC1839) showed increased kon values for RT wt compared to 2,6 F2analogs MC1236 and MC1281 (Fig. 2). 3000 K103N 2000 1500 1176.5 878.8 1000 500 0.0040 Y181I 296.3 223.5 319.5 0.0008 0.0000 0 MC1236 MC1281 MC1838 MC1236 MC1839 Concerning the mutants, all the inhibitors exhibited higher kon levels for E and E:NA, with respect to those for E:NA:dNTP. On the other hand, significantly low koff values were obtained for this latter catalytic complex, revealing major stability for the inhibitor binding to the enzymes when these are already assembled with both their substrates. MC1839 Relative Dissociation Index (RDI) koff mut / koff wt 85.00 95.00 80.00 -fold dissociation In conclusion, 2,6-Cl,F derivatives were able to bind to all the enzymes with lower dissociation rates than 2,6 F2-analogs: in particular, mutant and wt koff values appeared quite similar for MC1838, while binding of MC1839 to the mutants proved even more stable than to wt. MC1838 Figure 3: Relative Association Index (RAI) Figure 2: Association Rates In order to determine the basis for the observed resistance of mutants, we compared the kon and koff rates for the mutant ternary complexes to those for wt, calculating the relative association (RAI) and dissociation (RDI) indexes. All the compounds showed a drastic decrease in the association rates to the mutants, although this behavior appeared less marked for K103N. On the other hand, our results confirmed the clear correlation between the lower affinity towards the mutants and the moderate binding velocities observed for these inhibitors. MC1281 65.00 50.00 35.00 K103N 26.67 Y181I 20.00 5.00 1.38 0.79 0.13 0.05 MC1838 MC1839 -10.00 Figure 4: Relative Dissociation Index (RDI) MC1236 MC1281 DISCUSSION AND CONCLUSIONS RT HIV-1 antiviral research is currently orientated towards the discovery of novel molecules mainly effective against RT mutants rapidly selected in the course of the chemotherapy with NNRTIs. In particular, this study has been aimed to the identification of compounds active able to inhibit the most common mutant forms of the enzyme, carrying the amino-acidic substitutions K103N and Y181I. We assayed, therefore, some N,N-DABO derivatives, selected for their broad activity spectrum against NNRTIs resistant mutants. Previous molecular modelling studies, performed on the analogs S-DABOs and NH-DABOs, demonstrated that a great number of contacts between the DABO ligands and the RT NNBS residues occur, suggesting that tighter binding and increased activity for these compounds was due to a novel hydrogen bond, formed with the residue K101, with respect to other NNRTI classes. Though N,N-DABOs missed the opportunity to estabilish this latter hydrogen bond, they generally presented a major flexibility that allowed an easier binding also to mutated conformations of the allosteric site. In this regard, it was previously reported that the N,N-2,6 F2-analogs MC1236 and MC1281 showed a slow-binding tightbinding kinetic. In order to obtain inhibitors characterized by a faster binding mechanism, we considered novel 2,6-Cl,F-derivatives and observed higher association rates for these compounds, especially towards the free enzyme and the binary catalytic complex. Interestingly, MC1838 and MC1839 were greatly potent against K103N and Y181I, being their binding to the NNBS highly tight, although it appeared clearly more stable towards the ternary complex than towards the free enzyme and binary complex. Our results indicated that these compounds could find significant clinical applications in the antiviral therapy, though further toxicity and pharmacologic studies are needed. These data would be useful for further antiviral molecular modeling investigations.