Nomenclature & Formula Writing Chemistry Worksheet
advertisement

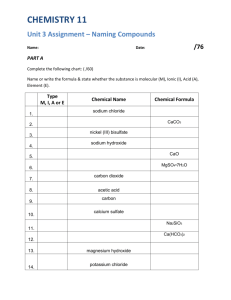
Nomenclature and Formula Writing Ionic Compounds (metal + nonmetal) ex. Binary Compounds (two elements only) - write the name of the metal (positively charged) - then write the name of the nonmetal using the ide ending. Compounds with Polyatomic Ions - write the name of the metal or positively charged ion - write the name of the complex ion ex. METAL NONMETAL STEM + IDE NaCl sodium chloride MgBr2 magnesium bromide METAL COMPLEX ION CaSO4 calcium sulfate NaHCO3 sodium bicarbonate or sodium hydrogen carbonate Multivalent Metals - Compounds where the metal has more than one oxidation number write the name of the metal write the oxidation number using roman numerals in parentheses o or write the name with the "ic" (higher oxidation #) or "ous" (lower oxidation #) ending. Some metals use their Latin names with these endings. write the nonmetal or the complex ion ex. Cu2O copper(I) oxide cupric Cu2+ cuprous Cu+ or ferric Fe3+ ferrous Fe2+ cuprous oxide auric aurous CuO copper(II) oxide Au3+ Au+ plumbic Pb4+ plumbousPb2+ or cupric oxide stannic Sn4+ stannous Sn2+ mercuric Hg2+ mercurous Hg+ Molecular Compounds (Covalent Compounds) (nonmetal + nonmetal) because nonmetals combine in more than one ratio, we must use prefixes to indicate the number of atoms of each element in the formula. the following prefixes are used: if the prefix is followed by a vowel, the final "a" or "o" is dropped exception: the prefix mono is omitted for the first element only. 1 ex. mono- 3 2 di4 N2O dinitrogen monoxide tritetraN2O5 5 penta- 6 hexadinitrogen pentoxide 7 hepta- 8 octaCO carbon monoxide 9 nona- 10 decaCO2 carbon dioxide Hydrates - Ionic Compounds There are many compounds that crystallize from a water solution with water molecules adhering to the particles of the crystal. These hydrates, as they are called, usually contain a specific ratio of water to compound. Chemists use heat to dry these compounds and then calculate the ratio of compound to water. An example of a hydrate is NiSO3•6H2O. The dot shows that 6 molecules of water adhere to 1 formula unit. To name the compound, name the portion preceding the dot, followed by the prefix for the number and hydrate. The above compound would be named: NiSO3•6H2O nickel(II) sulfite hexahydrate Acids Acids are a group of compounds that are given special treatment in naming. Acids are defined in several ways, but in general, we can say that acids are compounds that give off hydrogen in water. The formula of an acid is one or more hydrogens bonded to a monatomic or polyatomic anion. The way that the acid is named is determined by the suffix of the anion. hydrogen hydrogen hydrogen ide ate ite becomes becomes becomes hydro _ic acid ic acid ous acid examples: HCl HClO4 HClO3 HClO2 HClO hydrogen chloride hydrogen perchlorate hydrogen chlorate hydrogen chlorite hydrogen hypochlorite becomes becomes becomes becomes becomes hydrochloric acid perchloric acid chloric acid chlorous acid hypochlorous acid 1. Binary Ionic Compounds. Give the correct names for each of the compounds listed below. a) NaCl n) ZrS2 b) FrBr o) AgI c) KF p) BaSe d) RaS q) MgO e) LiI r) LaBr3 f) Li3N s) Sr3N2 g) AlBr3 t) Cd3As2 h) CdCl2 u) Rb2Se i) K2O v) Rb3N j) InF3 w) BaF2 k) ZnO x) ZrTe2 l) Y2O3 y) Cs3P m) CaTe z) Y2O3 2. Binary Ionic Compounds. Write the correct chemical formula for each of the following compounds. a) potassium bromide n) potassium nitride b) zinc bromide o) aluminum bromide c) lithium iodide p) zinc phosphide d) scandium chloride q) magnesium sulfide e) magnesium chloride r) hafnium chloride f) magnesium oxide s) barium sulfide g) hydrogen sulfide t) tantalum oxide h) gallium iodide u) zirconium nitride i) sodium oxide v) potassium selenide j) magnesium selenide w) germanium fluoride k) calcium fluoride x) francium phosphide l) aluminum oxide y) zinc arsenide m) beryllium chloride z) scandium telluride 3. Polyatomic Ions. Give the correct names for each of the compounds listed below. a) CaSO4 n) Ta(IO3)5 b) Ca3(AsO4)2 o) (NH4)3PO4 c) NH4Cl p) AgClO d) Mg3(AsO3)2 q) KOH e) NaC2H3O2 r) NaC8H11N2O3 f) NaOCN s) HNO3 g) Al2(SO4)3 t) In(VO3)3 h) K2Cr2O7 u) Na2HPO3 i) NH4NO3 v) Ta2(TeO4)5 j) KSCN w) Ca(NO)2 k) Al(OH)3 x) Zn(VO3)2 l) MgS2O8 y) Ba(OH)2 m) NaHCO3 z) CaC8H4O4 4. Polyatomic Ions. Write the correct chemical formula for each of the following compounds. a) sodium acetate n) silver fluorite b) aluminum tetraborate o) scandium hydroxide c) calcium bromate p) aluminum citrate d) sodium silicate q) hafnium nitrate e) magnesium citrate r) francium hydrogen oxalate f) calcium tungstate s) rubidium permanganate g) potassium cyanide t) gallium sulfite h) zinc phthalate u) ammonium dichromate i) barium carbonate v) cesium hypochlorite j) indium stearate w) sodium phosphite k) calcium dichromate x) sodium dihydrogen phosphate l) yttrium tripolyphosphite y) sodium hydrogen phosphate m) zirconium bicarbonate z) zirconium uranate 5. Multivalent Metals. Give the correct names for each of the compounds listed below. a) FeI3 n) PuPO4 b) Bi2(SO4)3 o) PdI4 c) FeI2 p) OsS2 d) HgHCO3 q) Co2S3 e) NiO r) Ti3N4 f) Pb(H2PO3)2 s) MnO2 g) CuBr2 t) NiSO4 h) Pt(CrO4)2 u) Ti(Cr2O7)2 i) Cr2O3 v) FeSO3 j) Sb2(SO5)3 w) Os(NO3)4 k) AuCl3 x) Hg(NO2)2 l) Np(MnO3)5 y) SnSO4 m) WO3 z) AuCl3 6. Multivalent Metals. Write the correct chemical formula for each of the following compounds. a) lead(IV) oxide n) polonium(IV) sulfide b) antimony(V) bromite o) vanadium(V) iodate c) cobalt(II) fluoride p) plumbic phosphate d) ferric thiosulfate q) molybdenum(VI) benzoate e) copper(II) cyanide r) niobium(V) oxide f) stannic tartrate s) aurous silicate g) copper(I) nitride t) titanium(IV) sulfite h) platinum(IV) dichromate u) cobaltous chloride i) nickel(II) acetate v) samarium(III) nitrite j) tin(II) peroxydisulfate w) plumbic hydroxide k) gallium(III) acetate x) terbium (IV) periodate l) gold(III) uranate y) iridium(IV) periodate m) osmium(IV) sulfate z) stannous bicarbonate 7. Molecular Compounds. Give the correct names for each of the compounds listed below. a) CS2 i) PBr5 b) SF2 j) N2O4 c) CO k) SO3 d) ICl3 l) SO2 e) CCl4 m) N2O3 f) As2O3 n) Cl2O g) PBr3 o) SF6 h) IF5 p) SiO2 8. Molecular Compounds. Write the correct chemical formula for each of the following compounds. a) nitrogen monoxide i) dinitrogen tetroxide b) carbon dioxide j) diphosphorus trisulfide c) iodine monochloride k) chlorine dioxide d) sulfur trioxide l) silicon disulfide e) chlorine trifluoride m) silicon tetrafluoride f) phosphorus pentachloride n) sulfur dioxide g) bromine pentafluoride o) tricarbon disulfide h) carbon tetrachloride p) dinitrogen pentoxide 9. Hydrates. Give the correct names for each of the compounds listed below. a) Li2SiF6•2H2O f) MgSO4•9H2O b) Na2B4O7•10H2O g) CaSO4•2H2O c) MgSO3•6H2O h) MgCl2•6H2O d) NaC2H3O2•3H2O i) FeSO4•7H2O e) CuSO4•5H2O j) NaHS•H2O 10. Hydrates. Write the correct chemical formula for each of the following compounds. a) calcium chloride hexahydrate f) plumbous acetate trihydrate b) barium chloride dihydrate g) aluminum chloride hexahydrate c) calcium nitrate tetrahydrate h) sodium dihydrogen phosphate nonahydrate d) sodium chromate tetrahydrate i) cobalt(II) nitrate hexahydrate e) copper(II) nitrate trihydrate j) cobaltous sulfate hexahydrate 11. Acids. Give the correct formula for each of the compounds listed below. a) hydrochloric acid n) hydroiodic acid b) citric acid o) phosphoric acid c) benzoic acid p) nitrous acid d) acetic acid q) thiosulfurous acid e) periodic acid r) nitric acid f) lactic acid s) hydrotelleric acid g) formic acid t) hydrocyanic acid h) iodic acid u) hydroselenic acid i) oxalic acid v) nitrous acid j) sulfurous acid w) hypooxalous acid k) sulfuric acid x) hydrofluoric acid l) carbonic acid y) boric acid m) phosphorous acid z) hydrosulfuric acid 12. Acids. Write the correct name for each of the following compounds. a) HC2H3O2(aq) m) HC5H8NO4(aq) b) H2B4O7(aq) n) H3PO4(aq) c) H3AsO3(aq) o) HClO(aq) d) HI(aq) p) HBr(aq) e) H3BO3(aq) q) H2C2O4(aq) f) HF(aq) r) H2CO3(aq) g) HCNO(aq) s) H2SiO2(aq) h) H2SO4(aq) t) HFO2(aq) i) H2C4H4O6(aq) u) HC17H35COO(aq) j) HCN(aq) v) H3PO3(aq) k) H(HCOO)(aq) w) HCl(aq) l) HNO3(aq) x) HBrO2(aq) A. Review. Give the correct chemical formula for each of the following compounds. 1. sodium hydroxide 35. nickel(II) peracetate 2. copper(II) sulfide 36. mercuric chloride dihydrate 3. potassium phosphide 37. dinitrogen trioxide 4. ozone 38. sodium hypoiodite 5. lithium nitride 39. potassium cyanide 6. lithium hydride 40. potassium aluminum sulfate 7. magnesium percarbonate 41. ammonium hypophosphite 8. aluminum sulfite 42. potassium uranate 9. sodium sulfate heptahydrate 43. lithium peroxide 10. sodium carbonite 44. perchloric acid 11. 45. ammonia perchloric acid 12. calcium hyponitrite 46. iodous acid 13. nitrous acid 47. hydrogen peroxide 14. sulfurous acid 48. gold(III) periodate 15. zinc acetate trihydrate 49. sodium oxide 16. potassium hypochromite 50. sodium glutamate 17. barium nitride 51. iron(II) sulfate 18. cobalt(II) perphosphate 52. barium perchlorate 19. carbon dioxide 53. manganese(II) nitrate 20. sulfuric acid 54. osmium(IV) thiosulfate 21. iron(III) chloride 55. chromium(III) nitrate 22. chromium(III) acetate 56. boric acid 23. hydrobromic acid 57. rubidium acetate 24. silver carbonate 58. hypoiodous acid 25. hydrogen bromide 59. cerium(III) phosphate 26. barium chloride 60. nitrous acid 27. boron trifluoride 61. chromium(III) nitride 28. calcium hydroxide 62. nitric acid 29. calcium hydride 63. magnesium nitrate 30. lead(II) hyposulfite 64. hypoiodous acid 31. hypophosphorous acid 65. copper(II) tartrate 32. carbonic acid 66. arsenous acid 33. beryllium perchlorate 67. magnesium hexafluorosilicate 34. ferrous hydroxide 68. cyanic acid B. Review. Give the correct names for each of the compounds listed below. 1. SnO2 35. Na3PO4 2. Sb2S3 36. Na2CrO4 3. HgS 37. LiClO4 4. MoS2 38. Zn(C2H3O)2 5. FeS 39. Au(CN)3 6. HgO 40. K2CrO4 7. AuCl3 41. KHCO3 8. NiBr2 42. Mn(OH)2 9. MgO 43. Ba(SCN)2 10. NaBr 44. RbCN 11. 45. NaBrO Al2O3 12. CaO 46. Al2(SO5)3 13. Ag2S 47. Fe(ClO)2 14. CaH2 48. (NH4)2CO3 15. K2CO3 49. Zn(NO2)2 16. (NH4)2S 50. Ca(NO3)2 17. Cr(NO3)2 51. NH4OH 18. KMnO4 52. NiPO2 19. SO3 53. NH3 20. P2S5 54. CaSO4 21. As2S3 55. Pb(HSO4)4 22. CCl4 56. Ca(ClO3)2 23. N2O4 57. AlPO4 24. NO 58. Li2CO2 25. H3BO3 59. PCl5 26. MgSCN 60. Mg(NO3)2 27. HNO2 61. SO2 28. As2S5 62. BaCr2O7 29. H3PO4 63. SrH2 30. Fe(NO3)2 64. H2SO4 31. H3AsO3 65. Na2O2 32. Cu2SO4 66. CsH2PO4 33. HIO3 67. Pb3(PO3)2 34. K2C2O4 68. HBr(aq)