Chem 167 Final Review

C HEM 167 F INAL R EVIEW
Part 2
R ESONANCE S TRUCTURES
Compound that cannot be represented by only one Lewis structure.
Determine resonance structures:
1) Ozone, O
3
2)
CO
3
2-
3) Benzene, C
6
H
6
S HAPES OF M OLECULES
Draw out the molecule in a Lewis dot structure
Pay attention to the lone pairs that could be present on the central atom.
Lone pairs push bonds closer together and farther away from the lone pair
Pay attention to if you are being asked for the electron configuration shape or molecular shape
An example of this would be NH
3 just by looking at domains it has a tetrahedral shape, but the lone pair makes the molecular shape trigonal pyramidal
H YBRIDIZATION OF M OLECULES
Rules for the number of hybrids created:
1) The number of hybrids is equal to the number of combined orbitals
2) There needs to be a hybrid orbital for each electron domain on the central atom
Examples: What type of hybridization is present?
1) O
3
2) H
2
S
3) CO
2
O VERLAPPING OF ORBITALS
Bonds are formed by the overlapping of orbitals also called constructive interference
These bonds formed are sigma and pi bonds
Sigma ( s) bonds are formed by s-orbitals overlapping and p-orbitals overlapping end-to-end
Pi ( p) bonds are formed by p-orbitals overlapping on their sides and also contains a sigma bond
A single bond is made of a sigma bond, a double bond contains a sigma and pi bond, and a triple bond contains 2 pi bonds and a sigma bond
Sigma bonds exist in the middle and pi bonds exist above and below the sigma bond
P OLARITY OF M OLECULES
A molecule is polar if there is a partially positive and partially negative area to it
Examples: CH
3
Cl, IF
5
, H
2
O
A molecule is nonpolar if its charges are balanced out and cancel
Examples: CO
2
, CH
4
Draw out these examples and see why they are polar or nonpolar
P HASE D IAGRAMS
Demonstrate how a substance changes with pressure and temperature
Know how to read a general phase diagram
(know the sections and what the lines represent)
Know the phase diagram for carbon, especially the split between solid carbon where it is diamond and graphite
C UBIC U NIT C ELLS AND HCP
HCP: hexagonal close packing, has max coordination number = 12; packing pattern:
ABAB
Simple cubic : packing efficiency is 1 atom, coordination number is 6 because it touches 6 other cells
Body-centered cubic: packing efficiency is 2 atoms, coordination number is 8
Face-centered cubic: packing efficiency is 4 atoms, coordination number is 12, which is the maximum coordination number close packed.
B AND D IAGRAMS AND P-N J UNCTIONS
Bands are made up of infinite atoms. Conduction band is made of anti-bonding orbitals. Valence band is made of bonding orbitals.
Metals (conductors): no band gap conduction
Semi-conductors: band gap, can be doped (p-type or n-type) to decrease this gap and allow conduction
P-type: on bottom of conduction band, dopant has less valence electrons than metal
N-type: on top of valance band, dopant has more valence electrons than metal
Insulator: huge band gap, cannot be doped, conductivity nearly impossible
D RAW B AND D IAGRAMS
1) Aluminum
2) P-doped Si
3) N
2
I NTERMOLECULAR F ORCES
Inside of a molecule not a bond
London dispersion forces: present in all molecules, due to electrostatic attractions
(random motion and temporary dipole)
Polarizability: greater in larger molecules because of more electrons and stronger dispersion forces.
Dipole-dipole: present in polar molecules, scales with molecular polarity, stronger than dispersion
Hydrogen bonding: between H and N,O, or F only, reason for water’s high BP
V APOR P RESSURE AND S URFACE T ENSION
Vapor pressure: equilibrium between evaporation and condensation, increases as temperature increases, weaker intermolecular forces lead to higher vapor pressure
Surface tension: due to intermolecular forces
Examples: meniscus vs. water droplet
Melting/Boiling point: low vapor pressure high
MP and high BP. High surface tension high MP and BP (from strong intermolecular forces)
P OLYMERS , P OLYMERIZATION , AND C OPOLYMERS
Polymerization: ways of creating polymers, you need to know two.
Addition polymerization: initiation step (free radical), propagation step (need C=C), termination step (combine free radicals)
Condensation polymerization: -OH of alcohol and
H combine to create H
2
O as byproduct
Polymer types: isotactic, syndiotactic, atactic
Copolymer types: alternating, block, graft
Additives: plasticizers, pigments, fire retardants, stabilizers
I NTERNAL E NERGY AND P-V W ORK
Made up of heat (q) and work (w), apply magnitude of vectors in a diagram
Heat: Exothermic is negative and heat/energy is released from system to surroundings.
Endothermic is positive and heat/energy is absorbed by system.
Work: Work is positive if the system is doing work. Work is negative if work is done on system by surroundings.
P-V work: If volume of products is greater than reactants then work is done by system and is positive. If volume is products is less than reactants then work is negative.
C ALORIMETRY
Calorimeter measures heat flow
2 Types
1) Constant pressure: coffee cup q calorimeter
= -q reaction
2) Constant volume: bomb q reaction q calorimeter q calorimeter
= Cv
= D E
D
= mc D T
T c = calorimeter constant reaction
Example: 1.435g C
10
H
8 is combusted in a bomb calorimeter what is D E reaction
T f
=25.95C Cv=10.17 kJ/C in kJ? T i
=20.28C and
P HASE C HANGES
Heating/cooling curve: areas of slope and latency
Slope: q=mc D T; c is dependent on stage of matter
Latency: where melting and vaporization occur q=n* D H vap/fus
Example:
How much energy (in kJ) is required to melt 150.0 g of ice from -18.00 C and bring the resulting liquid water up to
25.00 C? Specific heats: gas = 1.84 J/gC; liquid = 4.184
J/gC; solid = 2.09 J/gC.
D
H vap
= 40.7 kJ/mol
D
H fus
= 6.01 kJ/mol.
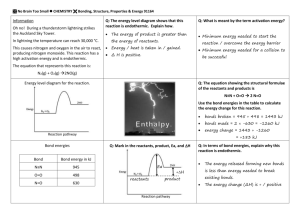
E NTHALPY /E NTROPY /G IBB ’ S F REE E NERGY
Enthalpy: measure of heat/energy. Positive = endothermic. Negative = exothermic
Entropy: measure of chaos or randomness of a system
Both are calculated as S n products
– S n reactants
Gibb’s free energy: measure of spontaneity of a reaction equal to D H – T D S. D G < 0 = spontaneous
Know the table of how the sign on D H and D S will give a spontaneous or nonspontaneous reaction or if it is spontaneous only as certain temperatures.
B OND D ISSOCIATION E NERGY
Standard enthalpy change in a reaction as reactants turn to products
Calculated: bonds broken – bonds formed
D H of bonds broken = positive because requires energy
D H of bonds formed = subtracted because gives off energy
Example:
Calculate the bond dissociation energy
H
2
(g) + Cl
2
(g) 2 HCl (g)
H—H:435kJ/mol, Cl—Cl:243kJ/mol, H—Cl:431kJ/mol
H ESS ’ S L AW
Way of finding the enthalpy of a reaction by applying and manipulating known enthalpy values of known reactions
Example:
Find the ΔH for the reaction below:
N
2
H
4
(l) + H
2
(g) 2NH
3
(g)
N
2
H
4
(l) + CH
4
O(l)
CH
2
O(g) + N
N
2
(g) + 3H
2
(g)
2NH
3
(g)
CH
4
O(l) CH
2
O(g) +H
2
(g)
2
(g) + 3H
2
(g) ΔH = -37 kJ
ΔH = -46 kJ
ΔH = -65 kJ
D ETERMINING R ATE L AWS
Instantaneous rate law: aA + bB cC
Rate = (1/c)( D [C]/ D t)=-(1/a)( D [A]/ D t)=-(1/b)( D [B]/ D t)
Rate expression: 2A + B A
2
B
Rate =
K[A] x [B] y
; x and y are the orders of the reactants, can only be determined through experiment
Overall order of a reaction is the sum of the orders of the reactants.
Example:
For the reaction A + B AB , the following data were obtained.
Trial Initial [A] Initial [B] Initial Rate
1 0.720 M 0.180 M 0.470
2
3
0.720 M
0.360 M
0.720 M 1.880
0.180 M 0.117
a) Determine the order with respect to each reactant b) Write the rate expression for the reaction.
c) Find the value of the rate constant, k.
I NTEGRATED R ATE L AW
First order: ln [A] t x-axis: t the slope=-k
= -kt + ln [A]
0, will produce a straight line on a graph with y-axis: ln [A] t and
Second order: 1/ [A] x-axis: t the slope = k t
= kt + 1/ [A]
0, will produce a straight line on a graph with y-axis: 1/ [A] t and
Third order: [A] t
= kt + [A]
0, x-axis: t the slope = k will produce a straight line on a graph with y-axis: [A] t and
Integrated rate laws are in y=mx + b format
H ALF LIFE OF REACTANTS
Zero order: t
1/2
= [A]
0
/2k
First order: t
1/2
= ln2/k = 0.693/k
Second order: t
1/2
= 1/k[A]
0
Questions given for these will be extremely straight forward and all you will need to do is insert values
A CTIVATION E NERGY AND A RRHENIUS E QUATION
K = A e ^ (-Ea/RT)
Use this modified version to find Ea: ln(K
2
/K
1
) = (Ea/R)(1/T
2
– 1/T
1
)
Again questions involving these equations will be straight forward. Just makes sure to keep the K and T values together that go in a pair.
R EACTION M ECHANISM
Mechanism is made up of elementary steps
Rate determining step: one step will be the slowest step and this is the rate determining step of the reaction
Molecularity: the molecularity of an elementary can be determined by the number of different species that make up the reactants.
Unimolecular>bimolecular>>termolecular
Example:
Write the total reaction, identify intermediates, and pick out rate determining step slow reaction: H
2
+ ICl HI + HCl k
1 fast reaction: HI + ICl I
2
+ HCl k
2
[H
2
] [ICl]
[HI] [ICl]
D YNAMIC E QUILIBRIUM
aA(g) + bB(g) cC(g) + dD(g)
K = K f
/K r
K c
= [C] c [D] d /[A] a [B] b Kp = P
C c P
D d /P
A a P
B b
K must be calculated at equilibrium and only for compounds in the gaseous or aqueous state
May need to construct an ICE table to calculate K
Example: Calculate K c
2HI 2H
2
(g) + 2I
2
(g) for the following reaction:
Start with 0.5M HI at equilibrium 0.0534M I
2
A CID I ONIZATION C ONSTANT
Calculated as [products]/[reactants]
Summed acid dissociation reaction = multiplied individual ionization constants
Can also find an elementary step constant in the total acid dissociation by dividing the quotient by individual constant(s)
R EACTION Q UOTIENT (Q)
Is calculated the same way as an equilibrium constant, but can be calculated with concentrations taken at any point in the reaction not just at equilibrium
If Q < K then reaction shifts to the left/reactants
If Q > K then reaction shifts to the right/products
L E C HATELIER ’ S P RINCIPLE
Provides ways that a system at equilibrium moves/shifts to offset a stress or disturbance on the system
Disturbances:
1) add/remove reactant or product
2) Change the volume or pressure changes moles of gaseous compounds
3) Temperature change exo: treat heat/energy as a product endo: treat heat/energy as a reactant
L E C HATELIER ’ S P RINCIPLE
N
2
O
4
( G ) 2NO
2
( G ) D H = 56.9J
1) NO
2 is added a. Equilibrium will shift to consume N
2
O
4
(g). b. Equilibrium will shift to produce more NO
2 c. Equilibrium will shift to consume the NO
2
(g).
(g). d. No effect on the equilibrium.
2) P is lowered by increasing V a. Produce more N
2
O
4
(g) to offset the pressure drop. b. Shift to the right to produce more NO
2 c . Shift to consume more NO
2
(g).
(g). d. No effect on the equilibrium.
3) Temperature is increased a. Equilibrium will shift to the left. b. Equilibrium will shift to the right. c. Equilibrium will shift to produce more heat. d. No effect on the equilibrium.
S OLUBILITY P RODUCT C ONSTANT
Constant is calculated as an equilibrium constant, use an ICE table
Solid salts are not included, acids are included
Can calculate pH from acid when K a is given [H+]
Change in the initial concentration (x) can be treated as negligible when calculating constant
Example:
Calculate M of Ag + ions in solution of Ag
2 with equilibrium [SO
4
2] = 0.1M K sp
SO
4
= 1.5 * 10 -5
C OMMON I ON E FFECT
If the same ion is added to a solution in which the ion is already present it decreases the solubility of the compound already present.
Example:
If NaCl is dissolved in solution determine which compounds will increase or decrease NaCl solubility a) NaNO
3 b) KBr c) CaCl
2 d) Li
2
SO
4
B RONSTED -L OWRY A CIDS AND B ASES
Acid: a proton donor
Base: a proton acceptor
Conjugate acid: acid formed from base’s accepted proton
Conjugate base: base formed by acid donating proton