Primer on Kinase Inhibitors
advertisement

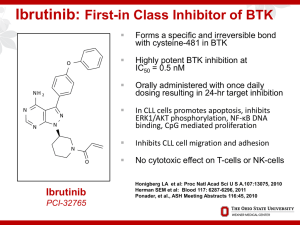
Ibrutinib: Analysis of Pivotal Data Richard R. Furman, MD CLL Research Center BCR-associated Kinases: Proven Effective Therapeutic Targets • Syk (spleen tyrosine kinase): 1. fostamatinib 2. PRT062070 3. GS-9973 • Btk (Bruton’s tyrosine kinase): 1. ibrutinib 2. CC-292 3. ACP-196 4. ONO-4059 • PI3K (phosphatidyl 3-kinase): 1. Idelalisib (GS-1101) 2. Duvelisib (IPI-145) 3. AMG319 Nat Rev Immunol 2:945 Targeting the “BCR++” Antigen Pathway: Issues with Novel Agents • Response Criteria – Redefine clinical endpoints – Evolution of response over time • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Re-evaluation of Prognostic Markers • Re-evaluation of MRD Redefining Clinical End Points “Cheson 2012” • Standard response criteria: measure of treatment efficacy • Need to provide means for determining need for treatment discontinuation • For novel agents, response criteria don’t measure effect: – Thalidomide / lenalidomide: tumor flare – BCR Antagonists: lymphocytosis (Not tumor flare) Cheson BD. JCO 2012;30:2820. Lymphocytosis + Nodal Reduction with BCR Antagonists Cheson 2012: Recommendations 1. For IMIDs: Assessment of PD should use repeat observations and incorporate indicators of PD not associated with tumor flares. 2. For BCR-targeted agents: lymphocytosis alone should not be considered an indicator of PD. Need to demonstrate other CLL-related signs or symptoms of PD. 3. Lymphocytosis is distinct from tumor flare Evolution of Responses Over Time: Kinase Inhibitors • Achievement of best response was time dependent • Proportion with CR/PR increased during follow-up • Proportion with PR+L diminished as the lymphocyte count declined over time Issues with Novel Agents • Response Criteria – Redefine clinical endpoints – Evolution of response over time • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Re-evaluation of Prognostic Markers • Re-evaluation of MRD Ibrutinib: Discovery Person Disease Enzyme Drug ibrutinib O NH2 N N N N N O Ogden Bruton (1908-2003) Bruton’s Agammaglobulinemia, 1952 Bruton Tyrosine Kinase, 1993 Synthesized 2005 First in human 2009 1st approval 2013 BCR Kinases All Interact With Each Other Ibrutinib: Inhibitor of Bruton’s Tyrosine Kinase • O • NH 2 N • • N N N N O • • Forms an irreversible bond with cysteine-481 in Btk Potent Btk inhibition IC50=0.5 nM Orally bioavailable Daily dosing resulting in 24-hr target inhibition Possible impact on T-cells Possible impact upon Tec, Bmx, Blk, Itk, and platelets Phase I Study of Ibrutinib in B-Cell Malignancies Cohort Dose No. of Patients CR PR I 1.25 mg/kg/d 7 0 2 II 2.5 mg/kg/d 9 1 3 III 5.0 mg/kg/d 6 2 1 IV 8.3 mg/kg/d 8 3 1 CD-I 8.3 mg/kg/d 10 1 5 V 12.5 mg/kg/d 7 0 4 CD-II 560 mg/d 9 1 6 56 8 (14%) 22 (39%) Total Advani RH. JCO 2013;31(1):88. IC50 Values of Ibrutinib and Related Kinases Irreversible Reversible Kinase Ibrutinib IC50 (nM) Kinase Ibrutinib IC50 (nM) BTK 0.46 FGR 2.31 BLK 0.52 CSK 2.30 BMX/ETK 0.76 Brk 3.34 EGFR 5.55 HCK 3.67 ErbB2 9.40 LCK 33.24 ITK 10.70 ABL 86.12 JAK3 16.13 Syk >10,000 TEC 77.76 JAK2 >10,000 Honigberg LA. PNAS 2010; 107:13075. BTK Inhibition and Plasma Levels 70 100% Plasma Concentration (ng/mL) Occupancy indicates irreversible inhibition of BTK 50 40 80% 70% Plasma Conc 60% % Active-Site Occupancy 50% 30 40% Plasma concentration profile reflects inhibition profile of reversibly inhibited off targets 20 30% 20% 10 10% 0 0% 0 4 8 12 16 Time Post-dose (h) 20 24 28 BTK Active-Site Occupancy 90% 60 Ibrutinib Pivotal Study: RESONATE R A N D O M I Z E Oral ibrutinib 420 mg once daily until PD or unacceptable toxicity n=195 1:1 IV ofatumumab initial dose of 300 mg followed by 2000 mg × 11 doses over 24 weeks n=196 Crossover to ibrutinib 420 mg once daily after IRC-confirmed PD (n=57) Eligibility: Relapsed and not appropriate for purine analog therapy: Disease progression < 3 years from prior purine analog Age >70 or age>65 with comorbidities Relapsed and deletion 17p purine analog associated AIHA / ITP Byrd JC. NEJM 2014; 371:213 RESONATE: Study Objectives • Primary Objective – PFS as assessed by the IRC per 2008 IWCLL criteria with the 2012 clarification for treatment-related lymphocytosis Secondary Objectives – Overall survival – IRC-assessed overall response rate – Safety and tolerability Exploratory Objective – Investigator assessed progression free survival and overall response rate RESONATE: Baseline Characteristics Ibrutinib (n=195) Ofatumumab (n=196) 67 (30-86) 67 (37-88) Male, % 66 70 purine analogs ref, % 45 45 ECOG PS 1, % 59 59 Rai stage III/IV, % 56 58 Bulky disease ≥5 cm, % 64 52 Del11q / 17p, % 32 / 32 30 / 33 # prior Rx, n 3 (1-12) 2 (1-13) 53 46 93 43 85 94 88 37 77 90 age (range), years ≥3 Prior therapies, % Prior therapy, % Alkylator Bendamustine Purine analog Anti-CD20 Byrd JC. NEJM 2014; 371:213 Patient Disposition Study treatment disposition Ibrutinib % Ofatumumab % Did not receive study drug Discontinued or completed Completed treatment Ongoing Median time on study, mos (range) 0 14 86 9.6 (0.33-16.62) 3 97 61 1 9.2 (0.07-16.49) Primary reason for discontinuation PD / Richter's AE Patient withdrawal Deaths Investigator decision 5/2 4 1 4 1 19 / 2 4 3 5 6 Byrd JC. NEJM 2014; 371:213 RESONATE: Progression Free Survival Progression-Free Survival (%) 100 90 80 70 60 Ofatumumab Ibrutinib Median PFS (mo) 8.08 NR Hazard ratio 0.215 (95% CI) (0.146-0.317) Log-rank P value < 0.0001 50 40 30 20 10 0 0 No. at risk Ibrutinib: 195 Ofatumumab: 196 3 6 9 12 15 38 15 7 1 0 Months 183 161 116 83 Ibrutinib significantly prolonged PFS 78% reduction in the risk of progression or death Investigator assessed PFS hazard ratio 0.133 (95% CI: 0.085-0.209) p value < 0.0001 Richter’s transformation was confirmed in 2 patients on each arm. An additional patient on the ibrutinib arm experienced disease transformation to prolymphocytic leukemia PFS By Prognostic Factor RESONATE: Overall Survival 100| | | | | 90 Overall Survival (%) Ibrutinib (n=195, 16 events) Ofatumumab (n=196, 33 events) |||||||||||||||||||||||| |||||||||||||||||| ||||||| || ||||| | | | || | || | | | | | || | | |||||||| ||||||| ||||||||||| || ||| ||| |||| ||| | ||| || || ||| | | | | | 80 70 Ofatumumab Ibrutinib Median time (mo) NR NR Hazard ratio 0.434 (95% CI) (0.238-0.789) Log-rank P value < 0.0049 60 50 40 30 20 10 0 0 3 6 9 Month 12 15 18 Ibrutinib significantly prolonged OS compared with ofatumumab This represents a 57% reduction in the risk of death for the ibrutinib arm At the time of this analysis, 57 patients initially randomized to ofatumumab were crossed over to receive ibrutinib following IRC-confirmed PD Resonate: Overall Response Rate Adverse Events in >15% Ibrutinib (n=195) Median treatment duration Any TEAE, % Diarrhea Fatigue Nausea Pyrexia Anemia Neutropenia Cough Thrombocytopenia Arthralgia Upper respiratory tract infection Constipation Infusion-related reaction Ofatumumab (n=191) 8.6 months 5.3 months Any grade Grade 3/4 Any grade Grade 3/4 99 48 28 26 24 23 22 19 17 17 16 15 0 51 4 2 2 2 5 16 0 6 1 1 0 0 98 18 30 18 15 17 15 23 12 7 10 9 28 39 2 2 0 1 8 14 1 4 0 2 0 3 Adverse Events in >15% Ibrutinib (n=195) Median treatment duration Any TEAE, % Diarrhea Fatigue Nausea Pyrexia Anemia Neutropenia Cough Thrombocytopenia Arthralgia Upper respiratory tract infection Constipation Infusion-related reaction Ofatumumab (n=191) 8.6 months 5.3 months Any grade Grade 3/4 Any grade Grade 3/4 99 48 28 26 24 23 22 19 17 17 16 15 0 51 4 2 2 2 5 16 0 6 1 1 0 0 98 18 30 18 15 17 15 23 12 7 10 9 28 39 2 2 0 1 8 14 1 4 0 2 0 3 AE: Bleeding • Resonate: all grades: 44% vs 12% grade 3-4: 1% vs 2% • BTK and TEK modulate glycoprotein VI signaling following binding of collagen • Three Studies: – Farooqui: PFA-100: epinephrine / ADP normal – Rushworth: aggregometry: collagen and ADP abnormal no explanation for ADP findings – Kamel: aggregometry: collagen abnormal AE: Diarrhea • Possibily mediated by EGFR inhibition • Reversible • Only symptomatic with food in GI tract • Take medication prior to bedtime SAEs / Atrial Fibrillation Ibrutinib (n=195) Ofatumumab (n=191) 8.6 months 5.3 months Subjects reporting ≥1 SAE 42% 30% Reporting ≥1 AE grade ≥3 57% 47% 24% 22% 5% 1% 3% 0% 44% 12% 1% 2% Adverse event, % Median treatment duration Any infection grade ≥3 Atrial fibrillation Grade ≥3 AE atrial fibrillation Any hemorrhage Major hemorrhage Conclusion • Ibrutinib initially approved based upon phase II data for relapsed CLL patients who have received at least one prior therapy • Based upon the RESONATE data, ibrutinib’s approval updated to include deletion 17p at any line of therapy • Phase III data provided new insights into adverse events: atrial fibrillation • Responses will evolve over time: STAY TUNED!