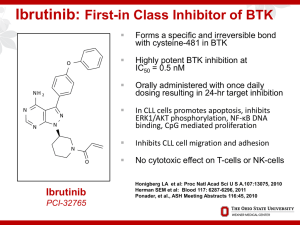
IDE1_v1.2_Idelalisib_rCLLwithRitux
advertisement
National Cancer Drugs Fund Application Form – Idelalisib In combination with rituximab for patients with chronic lymphocytic leukaemia (CLL) Author(s) David Thomson Owner Chemotherapy Clinical Reference Group Version Control Version Control Date Revision summary Ver0.1 14 Jul 2014 Draft for discussion Ver1.0 21 Oct 2014 Final version Ver1.1 12 Jan 2015 Removal of reference to interim access scheme Ver1.2 04 Nov 2015 Addition of criteria 7 Change to current version Criteria Changes 7 Addition of criteria regarding prior ibrutinib use National Cancer Drugs Fund – Application Form 04 November 2015 Idelalisib for relapsed CLL Page 1 National Cancer Drugs Fund Application Form – Idelalisib In combination with rituximab for patients with chronic lymphocytic leukaemia (CLL) Instructions to Consultants: Please fill in each section of the form electronically and save the document with your own file name. [If you continue typing the boxes will enlarge to contain the text]. Please send electronically to ______________________. Please also send copies to your Trust’s link accountant / corporate contracting team. Security of Patient Identifiable Information: The patient will be identified by their NHS number only. Please do not include any other patient identifiers for confidentiality reasons. All communication must be sent to the Cancer Drugs Fund Office via secure e mail accounts: that is from an nhs.net account to the ____________ account. Receipt of Application: The sender of the application will receive an acknowledgement, together with details of the unique Cancer Drugs Fund reference. Cancer Drugs Fund Policy: To check the status of a particular therapy please check the Cancer Drugs Fund Policy at _________________ Applications will be subject to Clinical Audit arrangements. BY TICKING THESE BOXES AND SUBMITTING THE APPLICATION THE CLINICIAN IS CONFIRMING THE PATIENT MEETS ALL THE CRITERIA BELOW. IT SHOULD BE NOTED THAT THE SACT DATASET WILL BE USED TO MONITOR THAT THESE CRITERIA ARE BEING MET. Approved Treatment Required for relapsed refractory CLL TICK All 7 conditions must be met 1. Application made by and first cycle of systemic anti-cancer therapy to be prescribed by a consultant specialist specifically trained and accredited in the use of systemic anti-cancer therapy 2. Confirmed CLL 3. Disease progression within 24 months of last systemic therapy 4. At least one previous anti-CD 20 antibody-based treatment or 2 previous chemotherapy regimens 5. Contraindications to cytotoxic chemotherapy (severe neutropenia or thrombocytopenia as a consequence of previous treatments) or an estimated creatinine clearance <60 mls/min or comorbidities as measured by a score of >6 on the Cumulative Illness Rating Scale 6. Given in combination with Rituximab at a dose of 375 mg/m2, followed by 500 mg per square meter every 2 weeks for 4 doses and then every 4 weeks for 3 doses, for a total of 8 infusions. Idelalisib should be continued to progression. 7. No prior treatment with ibrutinib unless ibrutinib has had to be stopped within 6 months of its start solely as a consequence of dose-limiting toxicity and in the clear absence of disease progression NOTE: Rituximab in this indication is funded via baseline commissioning Consultant Approval (email authority) Patient Consent Obtained (date of letter – copy to be retained on patient file) National Cancer Drugs Fund – Application Form 04 November 2015 Idelalisib for relapsed CLL Page 2 Proposed Start Date for Therapy (add clinic date)*: Consultant details* (including signature or email confirmation) Name: Hospital: Address: Post Code: Telephone: Nhs.net Trust Pharmacist details of the Trust where the patient will be treated* Mandatory - NHS No*: Mandatory – Patients date of birth* Optional – Hospital No. Clinical Commissioning Group* Patient’s GP* (name, address, telephone) Name: Hospital: Address: Post Code: Telephone: Nhs.net NHS No: DOB: Hospital No: CCG Name: Name: Address: Post Code: ICD-10 Code* C91.1 – Chronic lymphocytic leukaemia of B-cell type HRG Code Completion of items marked with * is mandatory. Failure to complete these items may mean that payment is not made. National Cancer Drugs Fund – Application Form 04 November 2015 Idelalisib for relapsed CLL Page 3