Primer on Kinase Inhibitors
advertisement
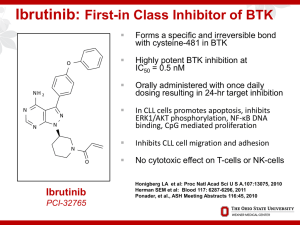
Primer on Kinase Inhibitors Richard R. Furman Directory, CLL Research Center Weill Cornell Medical College / New York Presbyterian Hospital BCR-associated Kinases: Proven Effective Therapeutic Targets • Syk (spleen tyrosine kinase): R406, PRT062070 • Btk (Bruton’s tyrosine kinase): ibrutinib, CC-292, ACP-196 • PI3K (phosphatidyl 3-kinase: idelalisib(GS-1101), IPI-145 Nat Rev Immunol 2:945 Targeting the “BCR++” Antigen Pathway: Novel BCR Acting Agents BTK: Ibrutinib (PCI-32765) CC-292 (AVL-292) ACP-196 PI 3 Kinase: Idelalisib (GS-1101, CAL-101) IPI-145 SYK: Fostamatinib (R935778) PRT062070 Issues with Novel Agents • Need to revise Response Criteria • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Differences Issues with Novel Agents • Need to revise Response Criteria • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Differences Lymphocytosis + Nodal Reduction with BCR Antagonists Redefining Clinical End Points “Cheson 2012” • Standard response criteria: measure of treatment efficacy • For novel agents, response criteria don’t measure effect: – Thalidomide / lenalidomide: tumor flare – BCR Antagonists: lymphocytosis (Not tumor flare) • Need to provide means for determining need for treatment discontinuation • LRF sponsored committee: May 2011 Cheson BD. JCO 2012. Cheson 2012: Recommendations 1. For IMID compounds: Assessment of PD should use repeat observations and incorporate indicators of PD not associated with tumor flares 2. For BCR-targeted agents: lymphocytosis alone should not be considered an indicator of PD. Need to demonstrate other CLL-related signs or symptoms of PD 3. Lymphocytosis is distinct from tumor flare Issues with Novel Agents • Need to revise Response Criteria • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Differences Idelalisib Doses >150 mg BID Associated with Longer PFS PFS -- By Idelalisib Dosing Regimen % Progression-Free 100 75 50 25 0 0 2 4 6 8 10 12 14 16 Cycles (28 days) 150-350 mg BID: 18 cycles (39) 50-100 mg BID: 5 cycles (16) 18 20 22 24 Issues with Novel Agents • Need to revise Response Criteria • Dosing: – No more MTD dosing – Threshold dosing – Fixed dosing / wide therapeutic window • Differences IC50 Values of PCI-32765 and Related Kinases Kinase PCI-32765 IC50 (nM) Kinase PCI-32765 IC50 (nM) Btk 0.46 FGR 2.31 Ikt 10.70 Fyn 95.55 Bmx/Etk 0.76 HCK 3.67 TEC 77.76 Lyn 200.45 EGFR 5.55 ABL 86.12 JAK3 16.13 Brk 3.34 BLK 0.52 JAK2 >10,000 LCK 33.24 SYK >10,000 Bruton’s Tyrosine Kinase (Btk) B-cell antigen receptor (BCR) signaling required for B cell survival Bruton’s Tyrosine Kinase (Btk) is an essential element of the BCR signaling pathway Inhibitors of Btk block BCR signaling and induces apoptosis Ibrutinib: Inhibitor of Bruton’s Tyrosine Kinase • O • NH 2 N • • N N N N O • • Forms an irreversible bond with cysteine-481 in Btk Potent Btk inhibition IC50=0.5 nM Orally bioavailable Daily dosing resulting in 24-hr target inhibition No impact on T-cells or NK cells Possible impact upon bmx, blk, and platlets Ibrutinib in CLL: PCYC-1102 Furman RR. iWCLL 2013 PCYC-1102: Patient Demographics Characteristic Median age, years (range) ≥ 70 years, n (%) Male, n (%) Female, n (%) Prior Therapies, n (%) <3 >3 TN ≥ 65 Years n = 31 R/R n = 85 71 (65, 84) 23 (74) 66 (37, 82) 30 (35) 19 (61) 12 (39) 65 (76) 20 (24) NA Median = 4 (1-12) 24 (28) 61 (72) β2M > 3.0 mg/L, n (%) 8 (26) 39 (46) Rai stage III/IV, n (%) 17 (55) 52 (61) Prognostic markers, n (%) IgVH unmutated del(17p)+ del(11q)+ 15 (48) 2 (6) 1 (3) 65 (76) 29 (34) 29 (34) Furman RR. iWCLL 2013 PCYC-1102: Patient Disposition TN ≥ 65 Years n = 31 R/R n = 85 Median time on treatment, months (range) 21.3 (0.3, 26.6) 16.3 (0.3, 28.7) Median time on study, months (range) 22.1 (2.5, 28.9) 22.1 (0.7, 29) Patients still on treatment, n (%) 26 (84) 53 (62) Patients discontinuing treatment, n (%) 5 (16) 32 (38) Reasons for treatment discontinuation, n (%) AE Treatment-related AE Death due to AE Disease progressionb 2 (6) 1 (3) 0 1 (3) 10 (12) 1 (1) 1 (1)a 10 (12) 0 0 2 (6) 0 4 (5) 4 (5) 3 (4) 1 (1) SCT (while in response) Investigator decision (not SCT) Patient decision Lost to Follow-up aCryptococcal b7 pneumonia patients (1 TN and 6 R/R) had disease progression with Richter’s transformation Furman RR. iWCLL 2013 PCYC-1102: Overall Response • • Among those patients whose initial response was PR-L, the majority achieved classic response by iwCLL criteria: TN: 9/13 (69%) R/R: 38/49 (78%) Combined ORR + (PR-L) in TN (84%) and R/R (88%) Ibrutinib Pivotal Study Schema: PCYC-1112 Patients will be randomized 1:1 to either arm A or B Treatment Arm A: Ofatumumab IV 12 IV doses over 24 weeks or until PD Week 1: 300 mg initial dose Week 2 through 8: 2,000 mg (once weekly) Week 12, 16, 20 and 24: 2,000 mg (every 4 weeks) Treatment Arm B: Ibrutinib PO 420 mg (3 x 140mg) orally daily until PD PI 3 Kinase d Signaling in B Cells Stromal cell T-cell Signaling stimulus IL-6 BAFF IL-6R BCR CD40 BAFFR B-cell membrane LYN JAK TRAF6 CXCL12/13 CXCR4/5 gp130 gp130 JAK SYK LYN/SYK STAT PI3K Delta BTK PLC2 PKC T308 AKT STAT BTK PLC2 S473 NF-k pathway GSK-3 mTOR p70s6k elf4E Lannutti, B. Blood, 2011 Idelalisib: Specific Inhibitor of p110d Tyrosine Phosphorylation PI3K Isoforms Expression Broad Broad Leukocytes Leukocytes Gene KO effect Lethal Lethal Benign Benign Insulin signaling Angiogenesis unknown B-cell signaling, development & survival 2154 427 8 Physiological role IC50 (nM) Neutrophil, T-cell development 182 Phase I Study of Idelalisib in Patients with Hematologic Malignancies Previously treated hematologic malignancies: CLL (N=54) iNHL (N=30) MCL (N=21) DLBCL (N=9) myeloma (N=12) AML (N=12) Idelalisib 50 mg to 350 mg BID Continuous oral dosing (28-day cycles) 48 weeks Endpoints: • Phase 2 dose • Safety • Pharmacodynamics • Pharmacokinetics • Antitumor activity CLL Patients Treated with Idelalisib 150 mg BID 100 81% 72% Decrease by 50% of nodal SPD PR with lymphocytosis (Cheson 2012) PR by IWCLL criteria (Hallek 2008) Response Rate 80 60 33% 40 20 39% 0 Nodal Overall Response Response Brown J. ASCO 2013 0 60 -2 0 40 -4 0 20 -6 0 9 80 -8 0 0 2 4 6 8 12 16 20 24 32 40 M ean SEM , % 0 C h a n g e in S P D f r o m B a s e lin e A LC, M ean SEM , x10 / L CLL Patients Treated with Idelalisib 150 mg BID ALC SPD 48 T im e f r o m S t a r t o f Id e la lis ib , W e e k s Brown J. ASCO 2013 Single Agent Idelalisib in CLL Best On-Treatment Change in Tumor Size (ITT Analysis, N=55) +100 % Change in Lymph Node Area +75 +50 +25 0 -25 -50* -75 -100 Inevaluable (patients without a follow-up tumor assessment) Patients with del (17p) * Criterion for response [Hallek 2008] Brown J. ASCO 2013 120 110 110 90 70 100 P la te le t C o u n t ( N = 3 4 ) H e m o g lo b in ( N = 2 5 ) 50 90 6 A N C (N = 1 5 ) 4 2 0 0 2 4 6 8 12 16 20 24 32 40 48 9 0 C e ll N u m b e r , M e a n S E M , x 1 0 /L H e m o g lo b in , M e a n S E M , g /L Improvement in Baseline Cytopenias T im e f r o m S t a r t o f Id e la lis ib , W e e k s Brown J. ASCO 2013 Idelalisib in CLL Progression Free Survival Overall Survival P F S (N = 5 4 ) O S (N = 5 4 ) 100 75 % S u r v iv in g % P r o g r e s s io n - F r e e 100 50 25 75 50 25 0 0 0 6 12 18 24 30 36 42 0 6 12 18 24 30 36 42 T im e f r o m S t a r t o f Id e la lis ib , M o n t h s T im e f r o m S t a r t o f Id e la lis ib , M o n t h s Median PFS = 17.1 months Median OS not reached Brown J. ASCO 2013 Adverse Events (> 15%) and Selected Lab Abnormalities (N=54) AE, n (%) Any Grade (%) Grade 3 (%) Fatigue Diarrhea Pyrexia Cough Back pain Rash URI Pneumonia Night sweats Chills 17 (32) 16 (30) 16 (30) 13 (24) 12 (22) 12 (22) 12 (22) 11 (20) 10 (19) 9 (17) 1 (2) 3 (6) 2 (4) 2 (4) 0 0 0 10 (19) 0 0 13 (24) 10 (19) 1 (2) 1 (2) Laboratory abnormality, n (%) AST, increased* ALT, increased* *15 subjects total with transaminase elevations Brown J. ASCO 2013 Idelalisib + +B Response Rate 95% CI +R +BR 100 80 90% 79% 78% 78% OR LNR OR 87% 87% LNR OR 60 40 20 0 LNR LNR = Nodal Response OR = Response by IWCLL criteria (Hallek 2008) Coutre S. ASH 2012, Abs 191 Idelalisib Pivotal Study Schema: GS-US-312-0116 IPI-145: Potent Inhibitor of PI3K-d and PI3K Isoform PI3K-δ PI3K- Expression Primarily Leukocytes Primarily Leukocytes Biochemical Activity (KD) 23 pM 243 pM Whole Blood Assay (IC50) 96 nM Anti-FcƐR1 1028 nM fMLP • Potent oral inhibitor of both PI3K-δ and PI3K-γ • Selective for PI3Ks over other protein and lipid kinases • Inhibits malignant B‐ and T‐cell survival – Affects tumor cells directly – Disrupts tumor cell interactions within the microenvironment Patel et al ASCO 2013 IPI-145 Complete Inhibition of PI3K-d and >50% Inhibition of at Doses > 25 mg BID Patel. ASCO 2013. IPI-145: Clinical Response Best Observed Response (n) CR PR nPR SD PD Population Pts (n) Median Time to IWCLL Response (range) Overall CLL 22 0 12 7 2 1 1.9 (1.8, 5.6) ≤ 25 mg BID 19 0 10 7 1 1 2.9 (1.8, 5.6) CD38+ 5 0 2 3 0 0 5.5 (5.5, 5.6) 17p del 4 0 2 0 1 1 1.9 (1.8, 1.9) TP53 mut 6 0 4 2 0 0 2.9 (1.8, 4.7) 3 0 2 0 1 0 1.8 (1.8, 1.9) 75 mg BID PR + Nodal R = 19/22 (86%) Patel, et al. ASCO 2013