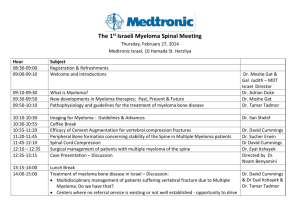
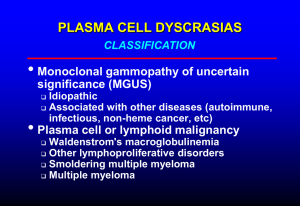
Rakesh Popat - UK Myeloma Forum
advertisement

Early Phase Myeloma Studies Rakesh Popat UCL Cancer Institute & University College Hospital Myeloma outcomes getting better… …but not good enough Kumar et al., Blood 2007 Kumar et al, Leukemia 2011 Why develop early phase trials in the UK? • Improve patient access to novel agents • Provide evidence to develop clinically relevant treatments for the UK • Influence drug development globally • Advance understanding of myeloma and drug resistance • Investigate treatment specific predictors and markers of response/ resistance Early phase clinical trials: the risks An approach to early phase trials Clinical Trial Network Academia Scientific question Pharma Novel drug(s) Early Phase Clinical Trial Trial Management Biomarker Evaluation Biological Markers of Activity Myeloma Treatments under evaluation Mahindra, A. et al. (2012); Nat. Rev. Clin. Oncol. doi:10.1038/nrclinonc.2012.15 UK Myeloma Phase I/II Studies Overview TYPE ACADEMIC 1st LINE PADIMAC RELAPSE MUK 6 COMMERCIAL C16006 CARDAMON MLN9708+MP MUK 5 DARATUMUMAB CVD vs CCarD +Rev Dex VTDPana PIPELINE +Vel Dex Vel Dex ± Tabalumab MUK 4 VelDex + Vorinostat MUK 3 CHR3996 + Tosedostat Proteasome Inhibitors K Anderson, ASH Education Book December 2011 New Diagnosis Velcade PAD = Doxorubicin Dexamethasone PAD (2-6 cycles) <PR ≥ VGPR PR Re-induction Stem cell harvest MRD Salvage High Dose Melphalan Relapse Observe High Dose Melphalan Can we defer high dose treatment in people that have responded well? PADIMAC MLN9708 with melphalan & prednisolone (phase 1/2) Days 1 4 8 15 22 28 INDUCTION 12 cycles • Newly diagnosed • Not suitable for ASCT MLN9708 Melphalan Prednisolone Days 1 8 15 22 MAINTENANCE up to 1yr MLN9708 28 MUK 5 (phase 2) CVD vs CCD Randomisation CVD 8 cycles 21 days • 1st relapse or refractory to 1 line of therapy • IMW Measurable disease CCarD 6 cycles 28 days No maintenance Maintenance Carfilzomib Up to 18 months Proteasome and HDAC Inhibition Hideshima T et al. Mol Cancer Ther 2011;10:2034-2042 MUK 6 (Phase 1/2) VTD-Panabinostat Days 1 3 5 8 10 12 21 INDUCTION 16 cycles Bortezomib Dexamethasone Panabinostat Thalidomide MAINTENANCE Panabinostat up to 1yr • 1- 4 prior lines • Prior BZ ok if responsive • Measurable disease (IMW criteria) MUK 3 (Phase 1/2) CHR3996 + Tosedostat Dose Level (DL) CHR-3996 (mg) Tosedostat (mg) 1 20 0 (nil) 40 0 (nil) 40 60 40 120 40 240 (dose reduction of 120 after 2 cycles) 2 3 4 5 Faith Davies Lab Monoclonal Antibody Targets Tabalumab Neri P et al. Clin Cancer Res 2007;13:5903-5909 Bortezomib +/- Tabalumab Phase 2 Daratumumab Daratumumab Fact Sheet Weers et al; The Journal of Immunology 2011;186(3) 1840-1848 Plesner et al., ASH 2012 Daratumumab & lenalidomide GEN503: A Phase 1/2 trial investigating the safety of daratumumab in combination with lenalidomide and dexamethasone in patients with relapsed or relapsed-andrefractory multiple myeloma van der Veer M S et al. Haematologica 2011;96:284-290 B Cell Receptor Signalling • • • • Ag induced aggregation of BCR – recruitment of SYC SYC phosphorylates BLNK – BTK – CARD11 CD19 activates PI3K Result activation of survival pathways Young & Staudt, Nature Reviews Drug Discovery 12, 229-243 (March 2013) Pathogenesis of Lymphoma BCR signalling in lymphoid malignancy • • • • • ABC DLBCL FL CLL MCL MAL • Burkitts • GCB DLBCL Young & Staudt, Nature Reviews Drug Discovery 12, 229-243 (March 2013) Targeting the BCR Young & Staudt, Nature Reviews Drug Discovery 12, 229-243 (March 2013) Weistner et al, Journal of Clinical Oncology, Vol 30, 2012 Ibrutinib & Myeloma (1) directly inhibit tumor growth (2) directly inhibit osteoclastic bone resorption, (3) inhibit the release of osteoclast-derived tumor growth factors (4) prevent adhesion to bone marrow stromal cells (BMSCs) and release of BMSC-derived growth factors. Edwards C M Blood 2012;120:1757-1759 Tai et al. Blood 2012;120(9):1877-1887. Ibrutinib Combinations Edwards C M Blood 2012;120:1757-1759 Tai et al. Blood 2012;120(9):1877-1887. Ibrutinib Combinations • • MYD88 L265P mutations cooperate with CD79B mutations to enhance BCR signaling addiction ABC DLBCLs with CARD11 mutations or MYD88 L265P without CD79B mutation resist ibrutinib Yang, Y. et al. Cancer Cell 2012 BCR inhibitor Myeloma Early Phase Trial Summary • • • • Number of early phase myeloma studies in UK Mix of commercial and academically sponsored Novel drug access to patients improving To be globally competitive: – Crucial to maintain innovative pipeline – Meet ambitious recruitment targets – Improve trial set up times