File - Groby Bio Page
advertisement

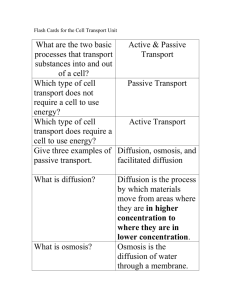
Exchanging Substances Facilitated Diffusion, Osmosis and Active Transport Passive Transport Learning Objectives • - explain what is meant by passive transport • - compare diffusion and facilitated diffusion • Identify the role of membrane proteins in transport Success Criteria • Construct definitions of diffusion, facilitated diffusion and osmosis • Create a comparison table • Draw and annotate diagrams to show how the processes differ Transport across the cell membrane All cells are surrounded by a partially-permeable membrane that controls what substances can enter and exit the cell. A cell needs to be able to import the substances it needs to survive, and to export waste materials and substances that are needed outside the cell. There are several methods by which substances (molecules and ions) can cross the cell membrane: diffusion osmosis active transport. What is diffusion? Diffusion is the net movement of particles down a concentration gradient: from a region of high concentration to a region of low concentration. net movement of particles No metabolic energy is expended during diffusion so it is an example of passive transport. One example of diffusion is gas exchange across respiratory surfaces, such as the lungs of mammals and birds, and the gills of fish. The rate of diffusion The rate of diffusion in a given direction across an exchange surface can be summarized by Fick’s law, which states that: surface area × difference in conc. rate of diffusion is proportional to: length of diffusion path (membrane thickness) Increasing the surface area across which the particles diffuse, or increasing the size of the concentration gradient will increase the rate of diffusion. Increasing the distance (or thickness of the membrane) over which diffusion takes place will decrease the rate. Plasma Membrane Plasma membranes form boundaries between cells and their environment. But substances need to cross membranes because: • Nutrients need to get in. • Waste/hormones etc. need to get out. OUTSIDE INSIDE Exchange Mechanisms Plasma membranes are partially permeable – they let some things through, but not others. The substances that can’t get through, need help crossing membranes. The ways in which substances can cross membranes are: Diffusion Facilitated Diffusion Active Transport Osmosis DIFFUSION Diffusion Recap All particles that make up liquids and gases are in random motion – they’re moving around very fast. Because of this, they move from: a region of high concentration to a region of low concentration High conc. Low conc. Diffusion Recap The molecules will diffuse both ways, but the net (overall) movement will be to the area of low concentration. There is a concentration gradient ..... particles move down it Diffusion is PASSIVE! There is NO energy involved. Diffusion occurs if the molecules involved can pass freely through a membrane. So they have to be small. If they’re not, facilitated diffusion is required! Rates of Diffusion The rate at which diffusion occurs is determined by several factors: 1. The size of the concentration gradient. The larger the difference in concentration, the faster diffusion will occur. 2. The thickness of the exchange surface. The thinner the exchange surface, the faster diffusion will occur. 3. The distance between the two areas. A shorter distance = faster diffusion. 4. The size of the molecules. Smaller molecules such as oxygen will diffuse quicker than large molecules like proteins. What is diffusion proportional to? surface area x difference in concentration length of diffusion path FACILITATED DIFFUSION Facilitated Diffusion Facilitated diffusion uses the same principle as ordinary diffusion, except that protein carriers are involved. Small molecules like O2 and CO2 can simply diffuse across a membrane without any help. Larger molecules like amino acids and glucose can’t diffuse directly through the phospholipid bilayer. They still move down a concentration gradient, but because they’re so big, they move through carrier proteins or channel proteins. Facilitated diffusion is also passive (no energy). CARRIER or transmembrane Proteins OUTSIDE INSIDE Carrier proteins move large molecules in or out of the cell down a concentration gradient. 1. Molecules attach to the carrier protein. 2. The carrier changes shape. 3. It releases the molecule on the other side. CHANNEL Proteins OUTSIDE INSIDE Channel proteins form pores in the membrane for CHARGED PARTICLES to move down a concentration gradient. Only open in response to presence of molecule Facilitated Diffusion • Facilitated diffusion is specific. • i.e. A certain type of molecule will have a corresponding carrier or channel Glucose = glucose channel Amino acids = amino acid channel ACTIVE TRANSPORT Active Transport Active transport is different from diffusion and facilitated diffusion because it uses ENERGY. Unlike diffusion and facilitated diffusion, molecules move AGAINST a concentration gradient. This happens in the intestines, where the concentration of nutrients is very high in the cells already. Active transport uses carrier proteins too, but they work using ATP (energy). Active Transport OUTSIDE ATP INSIDE 1. Molecule attaches to carrier protein. 2. ATP molecule provides energy to go against gradient. 3. Carrier changes shape and molecule is released on the other side (side with higher concentration). Cells like epithelial cells in the intestine have to carry out active transport. They’re packed with mitochondria to provide the ATP (energy) needed for transporting nutrients against a concentration gradient. Summary • Complete a table to show similarities and differences between the different methods of movement across a cell membrane Simple diffusion Energy required? Substances moving Location of cell membrane Factors affecting speed Facilitated diffusion Active transport Osmosis Starter • Name a cellular organelle that possesses a membrane and describe the purpose of the membrane. Golgi, mitochondria, chloroplast, nucleus, lysosomes Compartmentalise enzyme reactions/control substances in and out • Describe the purpose of cholesterol in the plasma membrane Prevents phospholipid tails from packing close together and preserves fluidity of membrane • Suggest why organisms living in polar regions have a high proportion of cholesterol in the membrane To keep membrane fluid and functioning correctly • List three substances that need to be transported into animal cells in order to survive. Oxygen, food, minerals, water • List two substances that need to be transported out of animal cells in order to survive Carbon dioxide, nitrogenous wastes OSMOSIS – TRANSPORTING WATER Osmosis Learning Objectives • - Explain what is meant by osmosis, in terms of water potential. • Success Criteria • Identify the direction of osmosis using water potential values (No calculations of water potential will be required); • - Recognise and explain the effects that solutions of different water potentials can have upon plant and animal cells. • Complete a quantitative practical task (practice) What is osmosis? Osmosis is the diffusion of water. It is the net movement of water molecules from a region of high water concentration to a region of low water concentration, through a partially-permeable membrane. net movement of water molecules Osmosis is the process by which cells exchange water with their environment, such as in the mammalian kidney. Osmosis Osmosis is simply the diffusion of water molecules. The definition for osmosis is: Water Potential • Water is rarely pure – it is never composed of 100% H2O molecules. • There are usually dissolved solutes in it such as minerals and ions. • Pure water would have a ‘water potential’ (Ψ) of zero. • But when there are dissolved solutes in water, there’s no longer 100% water molecules – so we say the water potential is more negative. 100% water Ψ = zero Ψ= more negative Ψ = even more negative Water Potential Water potential is measured in kilopascals (kPa). Water potential of pure water = 0 kPa Water potential of water with a pinch of salt added = -70 kPa Just remember that the more concentrated water is with solutes (substances), the more negative the water potential will be. What is water potential? The net movement of water by osmosis is determined by differences in water potential between two solutions connected by a partially-permeable membrane. Water potential is the tendency of water molecules in a system to move. It is denoted by the symbol Ψ and is measured in kiloPascals (kPa). Pure water has the highest water potential, and has a value of 0 kPa. Solutions have a lower water potential than pure water, and have a negative water potential. Water molecules always move from a region of high water potential to a region of low (more negative) water potential. Water movement during osmosis What is solute potential? The water potential of a solution is affected by the amount of solute it contains. The greater the amount of solute, the lower the water potential. free water molecule This is because water molecules bind to the solute molecules, reducing the number of water molecules that are free to diffuse. solute molecule The contribution that solutes make to the water potential of a solution is the solute potential (ΨS), and is a negative value. What is pressure potential? The water potential of a solution is also affected by the pressure applied to it. The greater the pressure, the higher the water potential. This is called the pressure potential (ΨP) and is always a positive value. In plant cells, the pressure potential is a result of the cell wall exerting pressure on the cytoplasm. Water potential is calculated using the following equation: water potential Ψ = solute potential + pressure potential = + ΨS ΨP Calculating water potential Osmosis in plant cells Osmosis in animal cells Water potential of potatoes Ψ = -40 kPa Ψ = -30 kPa Ψ = -36 kPa Understanding Water Potential • Kerboodle – 3.7 Maths Skills (Practice Questions) Annotate your diagram to explain what is happening Osmosis Osmosis in an Animal Cell • Copy and complete table – use a red blood cell as the example Water potential of external solution compared to cell solution Net movement of water State of cell Annotated diagram Higher (less negative) Equal Lower (more negative) Osmosis in an Animal Cell Water potential of external solution compared to cell solution Higher (less negative) Equal Lower (more negative) Net movement of water Enters cell Neither enters nor leaves Leaves cell State of cell Swells and bursts No change Shrinks Annotated diagram Contents of cell released Normal RBC Cell looks darker, as haemoglobin more concentrated. Cell shrunken and shrivelled. Ψ = -5 kPa Ψ = -2 kPa Ψ = -1 kPa Ψ = -3 kPa Ψ = -2 kPa Ψ = -4 kPa Draw the following diagrams, and show with arrows, which way you think osmosis will occur. Cells are affected by the water potential of their surroundings When you compare two water potentials, you can give special names to them in terms of how different they are: Ψ = -2 kPa Ψ = -2 kPa Ψ = -10 kPa Ψ = -10 kPa Ψ = -2 kPa Ψ = -2 kPa Cell is in a HYPOTONIC SOLUTION Cell is in a ISOTONIC SOLUTION Cell is in a HYPERTONIC SOLUTION PLENARY Exam Q’s 1. Diffusion is a passive transport process. What does this mean? 2. How does the thickness of an exchange surface affect the rate of diffusion across it? 3. What happens if a cell is placed in a hypotonic solution? Exam Q’s 4. Pieces of potato of equal mass were put into different concentrations of sucrose solution for three days. The difference in mass when they were taken out is shown in the table. Concentration of sucrose (%) 1 2 3 4 Mass difference (g) +0.4 +0.2 0 -0.2 a. Why did the pieces in 1% and 2% sucrose gain mass? b. Why did the mass of the piece in 3% sucrose stay the same? c. What happens to a cell placed in a hypertonic solution? PAGES 88-89