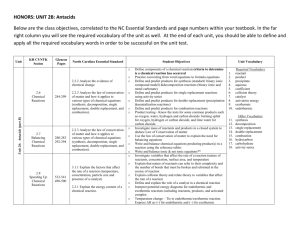
Single Replacement Reactions
advertisement

Types of Chemical Reactions Thermite reaction Types of Reactions • There are five types of chemical reactions we will talk about: 1. 2. 3. 4. 5. • Synthesis reactions decomposition reactions Single replacement reactions Double replacement reactions Combustion reactions You need to be able to identify the type of reaction and predict the product(s) Steps to Writing Reactions • Some steps for doing reactions 1. 2. 3. Identify the type of reaction Predict the product(s) using the type of reaction as a model Balance it Reminder! 1. 2. Don’t forget about the diatomic elements! For example, Oxygen is O2 as an element. In a compound, (MgO) it can’t be a diatomic element because it’s not an element anymore, it’s a compound! 1. Synthesis reactions • Synthesis reactions occur when two substances (generally elements) combine and form a compound. (Sometimes these are called combination or addition reactions.) SYNTHESIS GENERAL FORMULA reactant + reactant 1 product • Basically: A + B AB • • Example: 2H2 + O2 2H2O Example: C + O2 CO2 Synthesis Reactions • Here is another example of a synthesis reaction Practice • • • • Predict the products. Write and balance the following synthesis reaction equations. Sodium metal reacts with chlorine gas Na(s) + Cl2(g) Solid Magnesium reacts with fluorine gas Mg(s) + F2(g) Aluminum metal reacts with fluorine gas Al(s) + F2(g) 2. Decomposition Reactions • Decomposition reactions occur when a compound breaks up into the elements or into a few simpler compounds Decomposition GENERAL FORMULA • • • • 1 Reactant Product + Product In general: AB A + B Example: 2 H2O 2H2 + O2 Example: 2 HgO 2Hg + O2 Decomposition Reactions • Another view of a decomposition reaction: Decomposition Exceptions • Carbonates are a special case of a decomposition reaction that does not go to the elements. • Carbonates (CO32-) decompose to carbon dioxide and a metal oxide • • Example: CaCO3 CO2 + CaO There are other special cases, but we will not explore those in High School Chemistry Practice • • Predict the products. Then, write and balance the following decomposition reaction equations: Solid Lead (IV) oxide decomposes • • PbO2(s) Aluminum nitride decomposes • AlN(s) Practice Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: N2(g) + O2(g) (Nitrogen monoxide) BaCO3(s) Co(s)+ S(s) (Co+3) NH3(g) + H2CO3(aq) NI3(s) Warm-Up Sodium metal reacts with water to produce aqueous sodium hydroxide and hydrogen gas. Write the balanced equation for this reaction. 2Na(g) + 2H2O(l) 2NaOH(aq) + H2(g) Balance the equation C6H14 + O2 CO2 + H2O 2C6H14 + 19O2 12CO2 + 14H2O 3. Single Replacement Reactions • • Single Replacement Reactions occur when one element replaces another in a compound. A metal can replace a metal (+) OR a nonmetal can replace a nonmetal (-). Single Replacement General Formula • element + compound product + product A + BC AC + B (if A is a metal) CATIONIC OR A + BC BA + C (if A is a nonmetal) ANIONIC (remember the cation always goes first!) When H2O splits into ions, it splits into H+ and OH- (not H+ and O-2 !!) Single Replacement Reactions • Another view: Trade-off lab __Al(s) + __CuCl2(aq) → __AlCl3(aq) + __Cu(s) What type of reaction was the trade-off lab? Single Replacement Reaction Is it cationic or anionic replacement? General form: A + BC → AC + B Single Replacement Reactions Write and balance the following single replacement reaction equation: • Zinc metal reacts with aqueous hydrochloric acid Zn(s) + 2 HCl(aq) ZnCl2 + H2(g) Note: Zinc replaces the hydrogen ion in the reaction Is it cationic or anionic? Cationic • Single Replacement Reactions • Sodium chloride solid reacts with fluorine gas 2 NaCl(s) + F2(g) 2 NaF(s) + Cl2(g) Note that fluorine replaces chlorine in the compound • • Cationic or Anionic? Anionic 4. Double Replacement Reactions • Double Replacement Reactions occur when a metal replaces a metal in a compound and a nonmetal replaces a nonmetal in a compound Double Replacement General Formula • • compound + compound product + product AB + CD AD + CB Double Replacement Reactions • • • Think about it like “foil”ing in algebra, outside ions go together & inside ions go together Example: AgNO3(aq) + NaCl(s) AgCl(s) + NaNO3(aq) Another example: K2SO4(aq) + Ba(NO3)2(aq) 2 KNO3(aq) + BaSO4(s) Group Quiz Demo On a clean piece of paper, write both group members names Speculate as to why specks of solid are forming. One paragraph. Turn it into your folder Practice • Predict the products. Balance the equation 1. 5. HCl(aq) + AgNO3(aq) CaCl2(aq) + Na3PO4(aq) Pb(NO3)2(aq) + BaCl2(aq) FeCl3(aq) + NaOH(aq) H2SO4(aq) + NaOH(aq) 6. KOH(aq) + CuSO4(aq) 2. 3. 4. Warm Up 1. 2. Predict the Products and write the net ionic equation for the following reactions KI (aq) + Pb(NO3)2(aq) → A solution of calcium hydroxide is added to a solution of potassium sulfate Total Ionic Equations Once you write the molecular equation (Double replacement), you should check for reactants and products that are soluble or insoluble. We assume double replacement reactions are in water We can use a solubility table to tell us what compounds dissolve in water. If the compound is soluble (dissolves in water), then it splits the compound into its component ions If the compound is insoluble (does NOT dissolve in water), then it remains as a compound and the phase label is (s). Solubility Table Solubility Trends Gases only slightly dissolve in water Water slightly dissolves in water (H+ and OH-) Treat as insoluble Treat as insoluble Strong acids and bases dissolve in water Hydrochloric, Hydrobromic, Hydroiodic, Nitric, Sulfuric, Perchloric Acids Group I hydroxides Total Ionic Equations Molecular Equation: K2CrO4 + Pb(NO3)2 PbCrO4 + 2 KNO3 Soluble Insoluble Soluble Soluble Total Ionic Equation: 2 K+ + CrO4 -2 + Pb+2 + 2 NO3- PbCrO4 (s) + 2 K+ + 2 NO3- Net Ionic Equations These are the same as total ionic equations, but you should cancel out ions that appear on BOTH sides of the equation Total Ionic Equation: 2 K+ + CrO4 -2 + Pb+2 + 2 NO3- PbCrO4 (s) + 2 K+ + 2 NO3Net Ionic Equation: CrO4 -2 + Pb+2 PbCrO4 (s) Net Ionic Equations Try this one! Write the molecular, total ionic, and net ionic equations for this reaction: Silver nitrate reacts with Lead (II) Chloride in hot water. Molecular: Total Ionic: Net Ionic: 5. Combustion Reactions • • Combustion reactions occur when an element reacts with oxygen gas. This is also called burning!!! In order to burn something you need the 3 things in the “fire triangle”: 1) A Fuel 2) Oxygen to burn it with 3) Something to ignite the reaction (spark) Combustion Reactions In general: Hydrocarbon + O2 CO2 + H2O Or Element + O2 element oxide • Products in combustion are ALWAYS carbon dioxide and water or an oxide • 4th Period brainteaser Classify, Predict Products (if necessary), & balance 1. BaCl2(aq) + H2SO4(aq) 2. C6H12 + O2(g) 3. Zn(s) + CuSO4(aq) 4. K(s) + Br2(g) KBr(s) 5. H2O2(aq) O2(g) + H2O(l) 6. Na(s) + O2(g) Combustion Reactions Edgar Allen Poe’s drooping eyes and mouth are potential signs of CO poisoning. CO is a by-product of the combustion process if the hydrocarbon does not fully combust. Combustion • Example • • C5H12 + 8O2 5CO2 + 6H2O Write the products and balance the following combustion reaction: • C4H10 + O2 Activity Series of Metals Matter tends to react in such a way that more reactive substances form less reactive substances. Some metals are higher in reactivity than others Activity series: a list of metals arranged in decreasing reactivity (used for metal and hydrogen single replacement reactions) 42 5th period Warm-up Classify, Predict Products (if necessary), & balance 1. BaCl2(aq) + H2SO4(aq) 2. C6H12 + O2(g) 3. Zn(s) + CuSO4(aq) 4. K(s) + Br2(g) KBr(s) 5. H2O2(aq) O2(g) + H2O(l) 6. Na(s) + O2(g) I will go over the warm-up later in the period. On a blank piece of paper… Write one thing you know about chemical equations. Write one thing you need to know but struggle with about chemical equations Activity Series The higher the metal on the activity series, the more active that metal. Translation: higher metals on the chart will form ions in solution (dissolve in water…ie. Aqueous) the other metal in the reaction will precipitate (solid) Use the activity series on the back of periodic table to predict products and assign phase labels Predict whether a reaction occurs If a reaction occurs, predict the products. (don’t forget phase labels) Na (s) + H2O (l)→ Fe (s) + HCl (aq) → Fe (s) + Cr(NO3)3(aq) → Ni (s) + Pb(NO3)2(aq) → Ag (s) + Mg(NO3)2(aq) → Zn (s) + Co(NO3)2(aq) → Summary of Reactions For the test You should be able to …. Classify reactions Predict Products Write balanced molecular equations Write balanced net ionic equations Synthesis and Decomposition General forms: Synthesis A + B → AB Decomposition AB → A + B If you can’t see the general form of a reaction try labeling the reactants and products with “A” and “B”. Know how to classify these reactions Combustion Memorize the two general forms Hydrocarbon + O2(g) CO2(g) + H2O(l) Element + O2(g) element oxide Balancing the first general form can be tricky….. Just keep working at it. You must be able to classify and predict products in these reactions. Single Replacement Use the Activity series on the back of your periodic table to determine whether a reaction occurs. If a reaction occurs, predict products and balance….don’t forget phase labels. Classify as cationic or anionic General form: A + BC → AC + B (cationic) Or A + BC → BA + C (anionic) Double Replacement Predict products Use your Solubility Rules (pg 178) to determine whether a reaction occurs. If a reaction occurs, determine phases of products Determine the Net ionic equation if asked to do so. General Form: AB + CD → AD + CB From Warm-up 1. 2. 3. 4. 5. 6. BaCl2(aq) + H2SO4(aq) C6H12 + O2(g) Zn(s) + CuSO4(aq) K(s) + Br2(g) KBr(s) H2O2(aq) O2(g) + H2O(l) Na(s) + O2(g) Demo Gas Collection In your pairs, the person with the longest eyelashes go get your lab books on the back table. Technique for collecting gas via water displacement First reaction Zinc metal is placed in a jar. Hydrochloric acid is added to the jar Write the chemical equation for this reaction and classify it. Demo Gas Collection Technique for collecting gas via water displacement Second reaction Hydrogen Peroxide is poured into a jar. A catalyst (NaI) is then put into the jar. Hydrogen peroxide then breaks down into oxygen gas and liquid water. Write the chemical equation for this reaction and classify it. On a blank piece of paper… (5 minutes) What gas was formed in reaction #1? What happened when the burning splint was placed into the test tube? What gas was formed in reaction #2? What happened when the glowing splint was placed into the test tube? What did you learn from reaction #1? What did you learn from reaction #2? Demo Reaction #3 Solid calcium carbonate reacts with hydrochloric acid (HCl) Write an equation with your reactants Write an If….and…then… statement to test for which gas is produced. Demo What actually happened? What can you conclude about the gas being produced? Follow-up Write one thing you learned about chemical equations today. Write one thing you still need to know about chemical equations In pairs… The person with the brightest shirt go get a white board and dry erase marker. Taking turns answer the following questions. Hold the white board up to show me your answer. Question 1 Classify the following reactions 2NaBr + Ca(OH)2 CaBr2 + 2NaOH N2 + 3H2 2NH3 2KClO3 2KCl + 3O2 2NaCl + F2 2NaF + Cl2 CH4 + 2O2 CO2 + 2H2O 4P + 10O2 2P2O5 Question 2 If a reaction occurs, predict the products and balance FeCl3 + NaOH Zn + LiOH K + MgBr Question 3 Write the net ionic equations NaBr + CaF2 FeCl3 + NaOH Ca(NO3)2 + Na2SO4 Question 4 Balance a. __ HgO → __ Hg + __O2 b. __ Fe + __ O2 → __ Fe2O3 c. __ KClO3 → __ KCl + __ O2 d. __ Ca(OH)2 + __ H2SO4 → __H2O + __ CaSO4 e. __ Cu + __ AgNO3 → __ Cu(NO3)2 + __ Ag f. __ C2H6 + __O2 → __ CO2 + __H2O Warm-up Balance and Classify the following equation: Mg (s) + CO2 (g) + O2 (g) → MgCO3 (s) For Carbon dioxide, write the following items: Chemical formula Lewis dot structure VSEPR shape Polarity of bonds Overall polarity of the molecule Warm-Up 1. Write a balanced chemical equation for the following reactions Cu(NH3)4SO4 → solid Copper(II) sulfate + ammonia gas Aluminum + Fluorine gas → Aluminum fluoride 2. Classify these reactions Warm-up Predict products and classify these reactions Na2CO3(s) NH3(g) + H2SO3(aq) NF3(s) H2 (g) + O2 (g) Quiz Classify the reactions Predict the products (when necessary) and write a balanced chemical equation Write the net ionic equation when appropriate Don’t forget phase labels