Hypercalcemia
advertisement

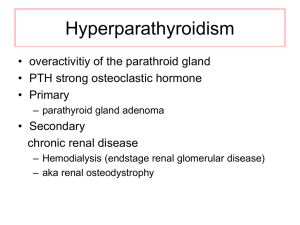
EMERGENCY TREATMENT OF SEVERE HYPERCALCEMIA Binu Abi, PharmD UW Medicine PGY1 Pharmacy Resident January 29th, 2015 Case • LC • 65 y/o M Wt 65 kg • CC • Presented to ED with complaints of dizziness, auditory/visual hallucinations, abdominal pain, polyuria in setting of starting duloxetine 10 days ago. • PMH • Alcohol abuse, depression, pancytopenia • Home Medications • Duloxetine • Allergies • Sulfa Case • Head CT – Normal • Vitals: normal • Labs Calcium • Essential for many important functions • Structure • Cell signaling • Regulation of protein function Intensive Care Med. 2013; 00(0):1-18. Calcium • 99% of total body calcium found in bone • 0.9% intracellular • 0.1% extracellular • Normal total calcium: 8.5 – 10.5 mg/dL • 50% bound to plasma protein (albumin) • Ionized or free calcium – physiologically active form regulated by homeostasis • Corrected calcium = (4- serum albumin) x 0.8 + serum calcium • Normal ionized calcium: 4.4 – 5.4 mg/dL Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Calcium Homeostasis • Calcium homeostasis is regulated by • Parathyroid hormone (PTH) • Vitamin D (Calcitriol) • Calcitonin Calcium Homeostasis – PTH • Secreted from parathyroid gland in response to drop in serum calcium • Increases serum calcium • Bone • resorption of calcium and phosphorus • Kidney • tubular reabsorption of calcium and phosphorus • vitamin D activity • GI tract • intestinal absorption of calcium and phosphorus (indirectly) Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Calcium Regulation – Vitamin D • 25 (OH) vitamin D – Calcidiol • Storage form • Liver • 1,25 (OH) vitamin D – Calcitriol • Active form • Activated by PTH and hypophosphatemia through 1-alpha hydroxylase in the kidney • Small intestine- absorption of calcium and phosphorous • Bone- osteoclast activity • Kidney- calcium and phosphate reabsorption Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Calcium Regulation – Calcitonin • Hormone produced by thyroid gland • Secreted when ionized calcium concentrations are high • Reduces serum calcium • Bone • osteoclastic resorption of calcium • Kidney • tubular reabsorption of calcium and phosphorus • GI tract • intestinal absorption of calcium and phosphorus (indirectly) Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Hypercalcemia • Elevated total serum concentration of calcium in blood • Mild • <12 mg/dL • Moderate • 12-14 mg/dL • Severe • >14 mg/dL • Hypercalcemia crisis • Acute elevation of total serum calcium > 15 mg/dL, acute renal insufficiency, obtundation Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Clinical Presentation • Varies depending on severity and rapidity of onset • Mild to moderate- often asymptomatic Manifestations of Hypercalcemia Neuromuscular Somnolence, confusion, depression, psychosis, coma, muscle weakness GI Constipation, anorexia, nausea, abdominal pain, peptic ulcer disease, pancreatitis Renal Decreased ability to concentrate urine, polyuria, polydipsia, nephrolithiasis, nephrocalcinosis, renal failure CV Hypertension, short QT, arrhythmias, digitalis sensitivity Skeletal Osteoporosis, fracture, bone pain Intensive Care Med. 2013; 00(0):1-18. Causes of Hypercalcemia • Parathyroid dependent • Primary hyperparathyroidism • Familial hypocalciuric hypercalcemia • Parathyroid independent • Malignancy • Medications • Paget’s disease • Endocrinopathies • Granulomatous disease • Hyperthyroidism • Pathophysiology • bone resorption • GI absorption • tubular reabsorption/ renal excretion Intensive Care Med. 2013; 00(0):1-18. Primary Hyperparathyroidism • Most common underlying etiology • Excessive secretion of PTH despite normal levels of calcium • 80% due to benign parathyroid adenomas • CKD secondary hyperparathyroidism • Usually presents as mild to moderate hypercalcemia Intensive Care Med. 2013; 00(0):1-18. Hypercalcemia of Malignancy • 20-40% of cancer patients experience hypercalcemia during course of disease • Tumors secrete PTH-related protein (PTHrP) increased bone resorption and renal tubular reabsorption • Most common in lung and breast carcinomas • 40% with multiple myeloma develop hypercalcemia • Usually presents as severe hypercalcemia Intensive Care Med. 2013; 00(0):1-18. Causes of Hypercalcemia – Medications Medication Mechanism Thiazides Increased renal tubular reabsorption Lithium Increased GI absorption increase bone resorption Increased renal tubular reabsorption Vitamin D Increased GI absorption Vitamin A Increase bone resorption Calcium Increased GI absorption Milk-alkali syndrome Aluminum/magnesium Antacids Theophylline Tamoxifen Ganciclovir Estrogen Pharmacotherapy: A Pathophysiologic Approach, 9e Complications of Hypercalcemia • Progression to hypercalcemia crisis • Renal failure • Coma • Life-threatening arrhythmias • Metastatic calcification • Osteoporosis Pharmacotherapy: A Pathophysiologic Approach, 9e Treatment of Severe Hypercalcemia • Intravenous Fluids • Diuretics • Calcitonin • Bisphosphonates • Others • Glucocorticoids • Cinacalcet • Denosumab • Hydroxychloroquine • Ketoconazole • Gallium nitrate Treatment – Intravenous fluids • MOA: expands intravascular space and increases renal excretion • Initial repletion reverses serum calcium 1-2 mg/dL • 3-6 L of NS during initial 24 hour period • Caution in CHF Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Treatment – Diuretics • Loop diuretics to promote calciuresis • Reduces calcium reabsorption in the loop of Henle • Furosemide 20-40 mg IV q2h after correction of dehydration • Effect on calcium levels: 2-6 mg/dL after 24 h • Limited evidence for use of diuretics for increased calciuresis • Excessive diuresis volume depletion, hypokalemia, and worsening hypercalcemia • Limit use to reversal of overly aggressive fluid replacement • Avoid thiazides! (enhances calcium resorption) Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Treatment – Calcitonin Calcitonin MOA Similar to human calcitonin; antagonizes the effects of parathyroid hormone. Inhibits osteoclastic bone resorption; promotes the renal excretion of calcium by decreasing tubular reabsorption Dose 4-8 IU/kg SC/IM q6-12 h Effect on Calcium Level 1-2 mg/dL Pharmacokinetics Onset: 2 h Duration: 6-8 h Metabolism: kidneys, blood, tissue Bioavailability: 71% SC, 66% IM Half-life: ~1 h Time to peak: ~30 min Adverse Effects Nausea, vomiting, flushing, injection site reactions Precaution Effective only up to 48 h – tachyphylaxis due to downregulation of receptors Miacalcin injection [prescribing information]. Novartis 2014. Treatment – Bisphosphonates • MOA: adsorb to the surface of bone and inhibit calcium release by interfering with osteoclast-mediated bone resorption • Zoledronic acid (Reclast, Zometa) – FDA approved for hypercalcemia • • • • • of malignancy (Zometa) Pamidronate (Aredia) – FDA approved for hypercalcemia of malignancy Etidronate (Didronel) Alendronate (Fosamax) Risedronate (Actonel) Ibandronate (Boniva) Intensive Care Med. 2013; 00(0):1-18. Treatment – Bisphosphonates Zoledronic Acid Pamidronate Dose 3-4 mg IV over 15-30 min x 1 60-90 mg IV over 2-24 h x 1 Dose for renal impairment Mild/moderate: no adjustment needed Severe: evaluate risk vs benefit, infuse longer Evaluate risk vs benefit, decrease dose and infuse over 4-6 h Effect on Calcium Level More potent than calcitonin, normocalcemia over 2-7 days PK Onset: 48 hours Duration: median 33 d Metabolism: not metabolized Half-life: 146 h Adverse Effects Flu-like symptoms (fever, arthralgia, myalgia, fatigue, bone pain), ocular inflammation (uveitis), hypocalcemia, hypophosphatemia, impaired renal function, nephrotic syndrome, osteonecrosis of the jaw Precaution Pregnancy Comments Bisphosphonates more efficacious than placebo (Saunders, 2004) Zoledronic acid superior to pamidronate (Major, 2001) Onset: 24-48 h Duration: 2-4 weeks Metabolism: not metabolized Half-life: 21-35 h Time to peak: ~30 min Pregnancy Treatment – Other • Infrequently used, specific disease states • Glucocorticoids – sarcoidosis, excess vitamin D • Cinacalcet – primary hyperparathyroidism, lithium • • • • associated hypercalcemia Denosumab – refractory to bisphosphonates Ketoconazole – sarcoidosis Hydroxychloroquine – sarcoidosis Gallium nitrate – multiple SEs Pharmacotherapy: A Pathophysiologic Approach, 9e Treatment – Other • Severely impaired renal function consider HD • Patients with primary hyperparathyroidism or malignancy may require surgery and or chemotherapy • Review patient’s medications and diet! • Hypercalcemia can increase risk of digoxin toxicity • Restore upright posture and mobility Pharmacotherapy: A Pathophysiologic Approach, 9e Summary of Treatment Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51 Back to LC • NS 2 L given in ED • Na 122, K 3.4, SCr 2.25, Ca 14 (corrected 14.3), albumin 3.6, PTH 7 • Calcitonin 260 units SC given (1/21/14 @ 1815) • Admit to medicine • Per patient- no appetite past week, took 20-30 tums daily for abdominal pain Diagnosed with milk alkali syndrome • Calcium carbonate and duloxetine held • Calcium continued to normalize (10.5 9.3 9.1) References 1. 2. 3. 4. 5. 6. 7. 8. Maier JD, Levine SN. Hypercalcemia in the intensive care unit: a review of pathophysiology, diagnosis, and modern therapy. Intensive Care Med. 2013; 00(0):1-18. Weiss-Guillet EM, Takala J, Jakob SM. Diagnosis and management of electrolyte emergencies. Best Pract Res Clin Endocrinol Metab. 2003; 17(4):623-51. Barton Pai A. Chapter 35. Disorders of Calcium and Phosphorus Homeostasis. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 9e. New York, NY: McGraw-Hill; 2014. Miacalcin injection [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; March 2014. Zometa [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; April 2014. Pamidronate Disodium [package insert]. Bedford, OH: Bedford Laboratories; October 2012. Saunders Y, et al. Systematic review of bisphosphonates for hypercalcemia of malignancy. Palliat Med. 2004;18(5):418-431. Major P, et al. Zoledronic acid is superior to pamindronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized controlled clinical trials. J Clin Oncol. 2001;19(2):558-567.