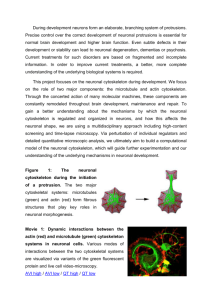
Neuronal Cytoskeleton14

Neuronal Cytoskeleton:
Structure and Function
Cytoskeleton
• Eukaryotic cell Skeletal System
– Three well defined filamentous structures
• Microtubules
• Microfilaments
• Intermediate filaments
Cytoskeleton
• Eukaryotic cell Skeletal System
– Micotubules
• Rigid tubes
• Tubulin
– Microfilaments
• Solid / thinner
• Actin
– Intermediate filaments
• Tough ropelike fibres
• Many related proteins
Cytoskeleton
• Functions of Cytoskeleton
– 1 Dynamic scaffold
– 2 Internal framework
– 3 Network of highways
– 4 Force generating apparatus – cell movement
– 5 m-RNA anchoring
– 6 Cell division
Cytoskeleton
• Microtubules – Structure and Composition
– Components of a diverse array of structures
• Mitotic spindle
• Core of flagella and cilia
– Tubes of globular proteins
• Longitudinal rows
• Protofilaments
• Cross-section – 13 rows of protofilaments – circular
– Dimeric building blocks – a
-tubulin and b
-tubulin
Cytoskeleton
• Microtubules – Structure and Composition
– a
-tubulin and b
-tubulin
• Similar 3-D structure
• Form dimers
• Fit together – non-covalent bonds
Cytoskeleton
• Microtubules – Structure and Composition
– a
-tubulin and b
-tubulin
• Linear array
• Asymmetric
• a
-tubulin at one end
• b
-tubulin at other end
• Same polarity
• Plus end = fast growing
• Minus end = slow growing
+ -
Components of Neuronal Cytoskeleton
(cont’d)
•
Microtubules
- Formed by 13 longitudinal strands arranged in helical configuration.
- Each strand is composed of aligned globular heterodimers consisting of α- and β-tubulin subunits.
- This leads to polarized assembly with one end having mainly exposed α subunits and the other end having mainly exposed β subunits.
Cytoskeleton
• Microtubules – Structure and Composition
– a
-tubulin
• Bound GTP
– Not hydrolysed
– Non-exchangable
– b
-tubulin
• Bound GDP
• Exchanged for GTP prior to assembly
The Cytoskeleton in Neuronal
Morphogenesis
•
Neurite (out)growth of growth cones.
- Occurs through the protrusion of filopodia and lamellipodia and the subsequent invasion of the expanded bases of filopodia and lamellipodia by MTs.
- The bundling of the invading MTs constitutes the consolidation of the growth of the neurite.
The Cytoskeleton in Neuronal
Morphogenesis (cont’d)
•
Axonal Maturation –
-When axons reach their targets, the cytoskeleton of the GC is remodeled and converted into the cytoskeleton of the presynaptic terminal.
- Motility and extension cease.
- Synapsin accumulates and cross-links synaptic vesicles to microfilaments.
Model showing the Origin of Axonal
Microtubules
The structure and action of growth cones
Components of Neuronal Cytoskeleton
(cont’d) Microtubules (cont’d)
Protein Assembly-Promoting and MT-Stabilizing
Property
•
HMW-τ
•
LMW-τ
• MAP-1A
•
-1B
•
-2A, B
• -2C, D
• -4
•
DCX
•
LLS1
•
Present in PNS.
•
Abundant in axons of CNS; Contributes to MT stabilization
•
Abundant in dendrites and mature neurons
•
Present in both axons and dendrites; contributes to neural migration and initial neurite outgrowth.
•
Present in both soma and dendrites (spines too).
•
Present in axon, dendrites, and glial cells.
•
Present in glial cells and in immature neurons.
•
Contributes to neuronal migration.
•
Contributes to neuronal migration.
Components of Neuronal Cytoskeleton
(cont’d) Microtubules (cont’d)
Protein
•
CLIP-170
•
APC
•
EB1
•
Kinesins
•
Dyneins
•
Gephyrin
•
OP18/ stathmin
Microtubule end-binding Protein
•
Attachment of Microtubules to endosomes
•
Attachment of Microtubules to cell cortex
•
Attachment of Microtubules to cell cortex
Microtubule-Activated ATPases
• Move organelles from “minus” to “plus” ends.
• Move organelles from “plus” to “minus” ends.
Proteins Anchoring MTs to Membrane Receptors
•
Binds glycine receptors
Microtubule-destabilizing Proteins
•
Highly abundant – favours MT destabilization
Cytoskeleton
• Microtubule Associate Proteins (MAPs)
– Mostly in brain
– Exception – MAP4 – many cell types (non-neuronal)
• Domain attaches to microtubule
• Domain extends out – filament
• Various roles
– Cross-bridges connecting microtubules
– Increase microtubule stability
– Alter microtubule rigidity
– Alter microtubule rate of assembly
– Activity – phosphatases and phosphokinases
Cytoskeleton
• Microtubules – structural roles
– Determine cell shape
• Axons of nerve cells
– Internal organization
– Axonal transportation
• Materials moved from cell body – along axon
– Anteriograde
• From axon to cell body – endocytosis
– Retrograde
– Axons have microfilaments, intermediate filaments and microtubules
• Interconnected
Interactions Among Cytoskeletal Components
AL, axolinin (squid giant axon MAP); RB, actin MF-assoc domain; RA MT-assoc domain
PL, plasma membrane
Cytoskeleton
• Microtubules – structural roles
– Passive
– Tracks for many motor proteins
– Motor proteins use ATP
• Move cellular cargo
– Vesicles, Mitochondria, Lysosomes, Chromosomes
– Motor proteins – Three families
• Myosins
• Kinesins
• Dyneins
– Kinesins and Dyneins – move on microtubules
Cytoskeleton
• Motor proteins
– Move unidirectionally
– Stepwise
– Series of conformational changes
• A mechanical cycle
• Coupled to chemical cycle – Energy
– Steps –
» ATP binding to motor
» Hydrolysis of ATP
» Release of ADP and P i
» Binding of new ATP
Cytoskeleton
• Motor proteins
– Kinesin
• Tetramer
– 2 identical heavy and 2 identical light chains
• Functional domains
– Pair of globular heads
» Bind microtubule
» ATP-hydrolysing
– Neck / stem and tail
– Tail binds cargo
• Move toward plus end of microtubule
– Plus end directed
• Motor proteins
– Kinesin
Cytoskeleton
Cytoskeleton
• Motor proteins
– Kinesin
• Velocity proportional to [ATP]
• Move one heterodimer at a time (step)
• One head – always attached
• Heads are coordinated
– Each at different stages of chemical and mechanical cycles
– When one head binds
» Conformational change in adjacent neck region
» Swings other head forward
• Kinesin – ‘walks’ along microtubule
Common Properties of Kinesin
1. Structure
N-terminal globular head: motor domain, nucleotide binding and hydrolysis, specific binding sites for the corresponding filaments.
C-terminal: structural and functional role: myosins
2. Mechanical properties, function cyclic function and work: motor binding to a filament force dissociation relaxation.
1 cycle requires 1 ATP hydrolysis.
They can either move (isotonic conditions) or produce force (isometric conditions)
The ATP Hydrolysis Cycle
ATP Cycle
Attached
τ on
Detached
τ off
δ = WD = step size;
V = ATPase activity v = in vitro sliding velocity
Attachement
Power stroke
Back stroke
δ = working distance
Detachment
Cytoskeleton
• Motor proteins
– Kinesin
• One member of a superfamily of related proteins
– Kinesin related proteins – KRPs
– Kinesin-like proteins – KLPs
– > 50
– Heads similar
– Tails heterogenous – binding different cargoes
• Motor proteins
Cytoskeleton
– Kinesin-mediated organelle transport
• Kinesins aligned with plus ends away from nucleus
• Tend to move organelles in anterograde direction
• Motor proteins
Cytoskeleton
– Cytoplasmic Dynein
• Movement of cilia and flagella
• And ubiquitous motor protein in eukaryotic cells
• Huge - > 1.5 Mda
• 2 identical heavy chains
• Many intermediate and light chains
• Heavy chain
– Large globular head
– Force generating engine
– Minus end directed
Cytoskeleton
• Motor proteins
– Cytoplasmic Dynein – Two roles
• Force generation – spindle – mitosis
• Minus-end directed motor for Golgi Complex and vesicles
• Requires a sub-unit complex dynactin
1.1-MDal protein (10-11 pps), which include p150-Glued and the filament-forming actin-related protein (ARP1).
Dynactin and actin bind via the p150-Glued subunit.
So, dynactin increases the run length of the dynein-driven movements, acting as a processivity factor for the dyneindriven motor on the MT.
Components of Neuronal Cytoskeleton
•
Microfilaments
- Composed of polymerization of actin (α and
β monomers.
- Must bind ATP to polymerize.
Dynamics occur through the incorporation and release of tubulin heterodimers at the ends of polymer
Microfilament dynamics are also associated with the
Exchange of actin monomers at the polymer ends.
Note the replacement of subunits.
Myosin
The headgroup of mysosin walks toward the head group of the actin filament (microfilament)
Components of Neuronal Cytoskeleton
(cont’d)
•
Intermediate Filaments
–
About 12 different isoforms, based on sequence homologies.
–
Expression is developmentally dependent.
- Neural stem cells express nestin (Class VI).
- Before differentiation, neuroblasts and neurons express vimentin (Class III).
- See next slide for Table
Polymerization of Intermediate Filaments
Central rods of the α-helix are hydrophobic interX coiled-coil dimer:
Dimer tetramer
(antiparallel structure).
Tetramers are connected
Longitudinally
(protomers).
8 protofilaments
1 filament
Intermediate Filament Proteins
Mass (kDal) and Distribution
Class and Protein
I.
Acidic cytokeratins
II.
Basic Cytokeratins
III. Vimentin
Desmin
GFAP
Periferin
IV. NF-L
NF-M
NF-H
α-interferon (NF-/66)
V. Lamins
VI. Nestin
•
(40-64); Epithelial cells
•
(52-68); Epithelial cells
•
(55); Mesenchymal cells, immature neurons, glial cells
•
(53); Myocytes
•
(51); Astroglial cells
•
(57); PNS neurons
•
(68); Neurons
•
(145); Neurons
•
(200); Neurons
•
(66); CNS neurons
•
(66-72); All cells
•
(240); CNS neural stem cells
The Cytoskeleton in Neuronal Morphogenesis
(cont’d): Axonal Maturation (cont’d)
•
Myelination –
Characterized by the radial growth of the axon (increased diameter), which is because of increased neurofilament expression and its phosphorylation.
Next slide: Stimulation of axonal neurofilament phosphorylation by myelinating Schwann cells.
- Note the interaction between Schwann cell membrane and axonal membrane molecules triggering either the activation of a neurofilament kinase (k) or the inhibition of a phosphatase (P)
enhanced phosphorylaiton of the ‘tail’ domains of the NF-H and
NF-M.
Regulation of Myelination
• Lateral projections of the NF polymers and high degree of phos electrostatic repulsion
wide interfilament spacing and incr axonal calibre.
• In nonmyleinated axon segments, the activity of the phosphatase > kinase activity NF less phosphorylated narrower interfilament spacing and decreased axonal diam.
Neuronal Polarity
Dendrites Axons
•
Uniform calibre
•
Few branches
•
Lack polysomes
•
Little, if any, protein synthesis
•
Fast growth
•
Neurofilament abundant
•
Uniform polarity of microtubles
•
Narrow spacing between microtubules
•
Abundance of tau protein
•
Presence of αγ spectrin
•
Highly phosphorylated NF-M and NF-H
•
Tapered morphology
•
Highly branched
•
Presence of polysomes
•
Some protein synthesis
•
Slow growth
•
Abundance of microtubles
•
Mixed polarity of microtubules
•
Wide spacing between microtubules
•
Presence of MAP2A, B
•
Presence of αβ spectrin
•
Nonphosphorylated NF-M and
NF-H
Cytoskeleton in Neuronal Plasticity
• Dendritic spines as postsynaptic structures.
• Actin – provides the main structural basis for cytoskeletal organization within dendritic spines
(lack MTs and IFs).
• Actin rearranges in synaptic plasticity (neuronal connectivity).
• LTP of synapses in hipp DG assoc with phosphorylation of cofilin , which incr in f-actin within spines growth and strengthening of synapses.
• Cytoskeletal modifications also alter neuronal physiology through modulating nt receptors and ion channels, which are anchored to the membrane cytoskeleton.
Neurons are Highly Polarized Cells whose
Organelles and Proteins are Differentially
Distributed
•
The soma is the main site of macromolecule synthesis.
•
The dendrites contain free ribosomes and synthesize some of their proteins.
- mRNA trafficking and local protein synthesis in dendrites.
•
The axon, to a large extent, lacks protein synthesis machinery.
Axonal Transport Allows Bidirectional
Communication between the Soma and the
Axon Terminals
•
Fast anterograde axonal transport is responsible for the movement of membranous organelles from the soma towards the axon terminal, and allows for renewal of axon proteins.
- Recall the role of kinesin and ATP.
Retrograde axonal transport returns old membrane constituents, trophic factors, exogenous materials to the soma.
•
Dynein.
•
Mechanism that regulates the direction of vesicle movement.
•
Functions of retrograde transport.
Slow Anterograde Axonal Transport Moves
Cytoskeletal Proteins and Cytosoluble
Proteins
•
The different cytoskeletal elements are assembled and connected by bridges in soma.
•
Cytoskeletal proteins are transported in a soluble form or as isolated fibrils and assembled during their progression.
•
The transport of microtubles and neurofilaments is bidirectional, intermittent, asynchronous, and occurs at the fast rate of known motors.
Axonal and Dendritic Intraneuronal
Transport
• Slow Component A: Moves proteins at a rate of
0.2-1 mm day -1 ; Consists mostly of pps assoc with
NFs and MTs.
• Slow Component B: Comprises > 100 pps moving at 2-8 mm day -1 . Transport of MTs and actin filaments including their assoc proteins.
• Intermediate Component: Mitochondria conveyed along MTs at 50-100 mm day -1 .
• Fast Component: Complex group of membraneassoc proteins moving at 200-400 mm day -1 and corresponds to most membrane organelles along
MTs.