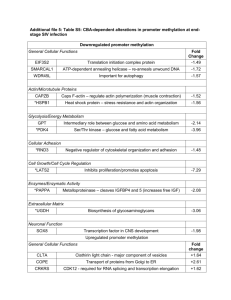
During development neurons form an elaborate, branching system
advertisement
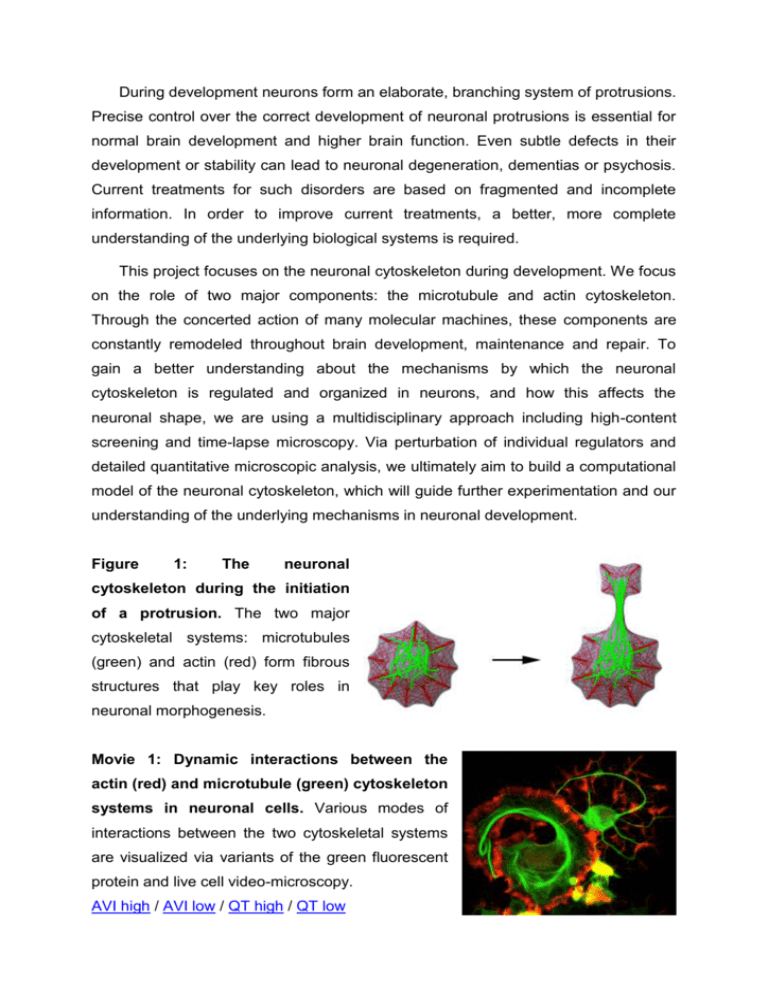
During development neurons form an elaborate, branching system of protrusions. Precise control over the correct development of neuronal protrusions is essential for normal brain development and higher brain function. Even subtle defects in their development or stability can lead to neuronal degeneration, dementias or psychosis. Current treatments for such disorders are based on fragmented and incomplete information. In order to improve current treatments, a better, more complete understanding of the underlying biological systems is required. This project focuses on the neuronal cytoskeleton during development. We focus on the role of two major components: the microtubule and actin cytoskeleton. Through the concerted action of many molecular machines, these components are constantly remodeled throughout brain development, maintenance and repair. To gain a better understanding about the mechanisms by which the neuronal cytoskeleton is regulated and organized in neurons, and how this affects the neuronal shape, we are using a multidisciplinary approach including high-content screening and time-lapse microscopy. Via perturbation of individual regulators and detailed quantitative microscopic analysis, we ultimately aim to build a computational model of the neuronal cytoskeleton, which will guide further experimentation and our understanding of the underlying mechanisms in neuronal development. Figure 1: The neuronal cytoskeleton during the initiation of a protrusion. The two major cytoskeletal systems: microtubules (green) and actin (red) form fibrous structures that play key roles in neuronal morphogenesis. Movie 1: Dynamic interactions between the actin (red) and microtubule (green) cytoskeleton systems in neuronal cells. Various modes of interactions between the two cytoskeletal systems are visualized via variants of the green fluorescent protein and live cell video-microscopy. AVI high / AVI low / QT high / QT low People involved in the project: Leif Dehmelt – principal investigator Julia Arens – PhD student Silke Gandor – PhD student Magda Krejczy – graduate assistent Anja Biesemann – master student Verena Hannak – master student Pia Jeggle – master student Relevant Publications: Halpain S, Calabrese B, and Dehmelt L. Actin Cytoskeleton in Growth Cones, Nerve Terminals, and Dendritic Spines. New Encyclopedia for Neuroscience, (Ed. Larry Squire) (in press). Dehmelt L, Halpain S. (2007): Neurite Outgrowth: A Flick of the Wrist. Curr Biol 17:R611R614 Dehmelt L, Nalbant P, Steffen W, Halpain S. (2006): A Microtubule-Based, DyneinDependent Force Induces Local Cell Protrusions: Implications for Neurite Initiation. Brain Cell Biology 35:39-56 Halpain S, Dehmelt L. (2006): The MAP1 family of microtubule-associated proteins. Genome Biology ;7:224. Dehmelt L, Halpain S. (2005): MAP2/tau family proteins. Genome Biology 6:204 Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. (2004): MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol 14:363-71 (featured in N&V, Nature Cell Biology 6:390) Dehmelt L, Halpain S. (2004): Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol 58:18-33 Dehmelt L, Smart FM, Ozer RS, Halpain S (2003): The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci 23:9479-90 (cover illustration)