Naming and Writing Formulas for Compounds
advertisement

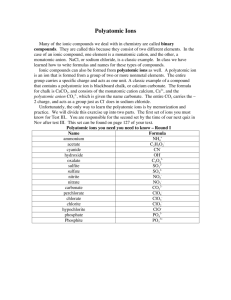
NAMING AND WRITING FORMULAS FOR COMPOUNDS Unit 4 1. NAMING IONIC COMPOUNDS • Ionic Compound: • Composed of ions • Cations & Anions • Metals & Nonmetals Cations: • + charge • Metals • + Polyatomic Ions Anions: • - charge • Nonmetals • - Polyatomic Ions 1. Name the cation (always 1st) 2. Name the anion – add “ide” ending (always 2nd) If anion is polyatomic, just use name (no ending) * This is for cations with a single charge (oxidation state) • For cations with more than one oxidation state • Name the cation • Add roman numeral to indicate charge of the cation 2. WRITING THE FORMULAS FOR IONIC COMPOUNDS 1. Write the cation symbol 2. Write the anion symbol (or polyatomic formula) 3. Balance + and – charges so they add to zero 3. NAMING AND WRITING FORMULAS FOR MOLECULAR COMPOUNDS • Molecular Compounds • Nonmetals only • Use greek prefixes to tell # of atoms 1 – mono 2 – di 3 – tri 4 – tetra 5 – penta 6 – hexa 7 – hepta 8 – octa 9 – nona 10 - deca 4. ACIDS • Recognizing an acid • Molecular compound beginning with “H” (or H2 or H3) • May contain polyatomic ions Binary Acids 1. Start name with “hydro” 2. Use anion name adding “ic” ending 3. End with “acid” Oxyacids 1. Use polyatomic ion name 1. Add “ic” in place of “ate” 2. Add “ous” in place of “ite” 2. End with “acid” Writing Acid Formulas 1. Write the symbol for hydrogen 1st 2. Write the symbol for nonmetal or polyatomic anion 3. Balance + and – charges so they add to zero • (H will have a charge of +1) 5. DIATOMIC MOLECULES • 2 of the same atom bonded together • Some elements naturally exist as diatomic H, N, O, F, Cl, Br, I 6. HYDRATES • Ionic salt with molecules of water attached Naming Hydrates 1. Name the ionic compound 2. Use a greek prefix for number of water molecules 3. End with “hydrate” Writing the Formulas for Hydrates 1. Write the formula for the ionic compound 2. Indicate the number of water molecules with big number in front of H2O 7. COMPOUNDS WITH COMMON NAMES 1. H2O 2. NaHCO3 3. H2O2 4. NH3 5. CH4 6. HCH3COO (HC2H3O2)