Lesson 12: potential difference
advertisement

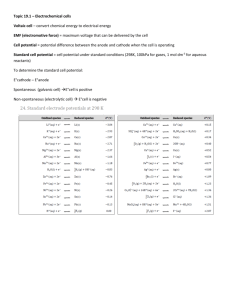
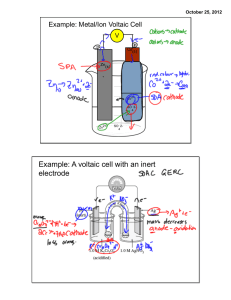
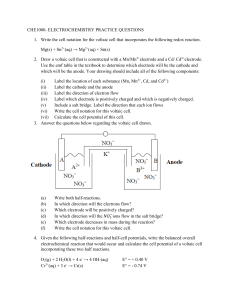
Cell Potential Objective lesson 3 chapter 13 You will be able to calculate the potential difference of a voltaic cell Standard Cell Definition A voltaic cell in which all of the electrolytic solutions are at 1.0 mol/L and they are at SATP. Recall SATP is 25˚C and 101.325 kPa. Symbol is E˚ It is a measure of the potential energy (voltage) of the electrons in the cell. It depends upon the · Make up of the half cells · Concentration of the electrolytes · temperature Reduction Half Potential Each half reaction in the data book has a standard reduction potential associated with it. (E˚R) This is a measure of the attraction for electrons. The stronger the OA, the higher the reduction potential. Cell Potential Cathode Positive electrode The half cell with the greater reduction potential. Anode Negative electrode Has lower reduction potential E˚cell = E˚cathode – E˚anode Example 1 What is the cell potential of a voltaic cell that is composed of a copper half cell and a silver half cell? Note A positive number indicates a spontaneous reaction. Example 2 What is the standard cell potential of a voltaic cell composed of cadmium and zinc half cells. Measuring Cell Potential Difference Potential differences are measured with a voltmeter. A voltmeter measures the difference in potential between two areas. This means that it must be measured across both half cells. Since a half cell cannot have a potential difference, it must be compared to a reference cell. Reference Half Cell Hydrogen was arbitrarily chosen as the reference point. You will notice from the listing of E˚ that the reaction that has a potential of zero is + 2H (aq) + 2e H2(g) Example 3 A hydrogen half cell is connected to an I2 | I-(iodine | iodide) half cell. Label the cathode, anode and write the half reactions. Example 4 Label the electrodes, write the half reactions, and calculate the cell potential for an aluminum and hydrogen cell. Think About It! If another element had been chosen as the reference cell, how would that affect the half cell potentials? How would it affect cell potentials? Example 5 Determine a revised set of cell potentials for copper, zinc, and hydrogen if copper is assigned a potential of zero. Cu2+ + 2e-➔Cu +0.34 V 0.00 V 2H+ + 2e- ➔ H 0.00 V 2 Zn2+ + 2e- ➔ Zn-0.76 V Assignment Do the worksheet. 0.00 V