No Slide Title
advertisement
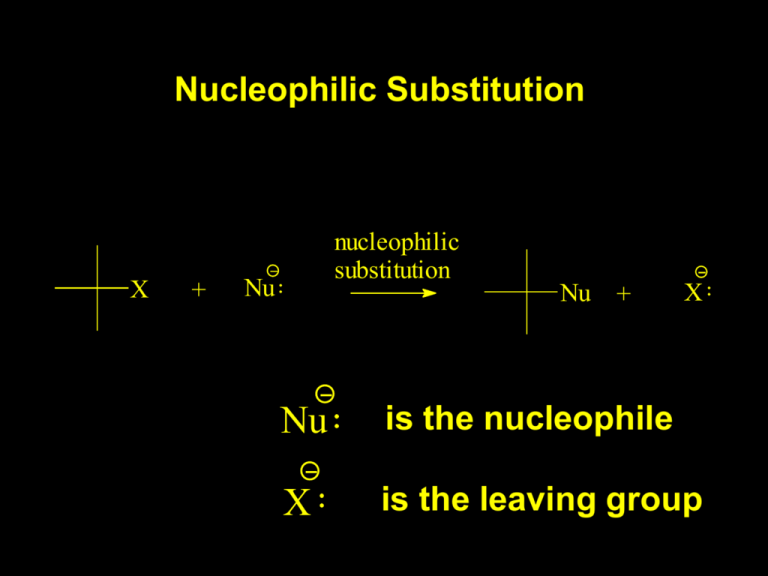
Nucleophilic Substitution X + nucleophilic substitution Nu Nu + X Nu is the nucleophile X is the leaving group nucleophilic substitution CH3 Br + Nu Nucleophile HO CH3O H3N CH3 Nu + Br Product CH3OH CH3OCH3 CH3NH3 H H2O CH3 O H Base CH3OH OH- Br OH NH3 Br NH3 But where do alkyl bromides come from? Br HBr But what about a primary bromide? OH HBr Nucleophilic Substitution works both ways! Br Mechanisms of Nucleophilic Substitution Reaction There is more than one. The mechanisms are determined by studying the rate of the reaction. A rate of a reaction refers to the change in concentration of the reactants or products versus time. First Case: CH3 Br + Nu Rate = - d [CH3Br] dt CH3 Nu + Br = k [CH3Br] [Nu] The rate that the CH3Br disappears is proportional to the concentration of the CH3Br and the concentration of the nucleophile. First Case: CH3 Br + Nu Rate = - d [CH3Br] dt CH3 Nu + Br = k [CH3Br] [Nu] The rate depends on the concentration of two components so it is a bimolecular reaction. First Case: CH3 Br + Nu CH3 Nu + Br The reaction is called an: SN2 reaction S for substitution N for nucleophilic 2 for bimolecular Transition State HO Br Ea HO- + CH3Br H HOCH3 + Br- HO H Br HH Transition State. Exists only for a very short time. The key step in the reaction is a collision between the two reactants. This means that an increase in the concentration of either reactant will result in a direct increase in the rate. Rate = - d [CH3Br] -] = k [CH Br] [OH 3 dt Second Case: Br (CH3)3CBr Kind of Strange HO OH + Br No substitution (CH3)3COH H2O OH + HBr (CH3)3COH No substitution with HO-, but reacts with H2O No substitution with HO-, but reacts with H2O HO- is much more basic than H2O. It should be a better nucleophile. Most interestingly the rate does not depend upon how much H2O is present. Rate = - d [(CH3)3CBr] dt = k [(CH3)3CBr] The rate does not depend upon concentration of H2O The key step in the reaction can not be bimolecular. It must be unimolecular. It turns out to be a multi step reaction: Step one is ionization to give the t-butyl carbocation and bromide. + Br (CH3)3CBr (CH3)3C CH3 CH3 CH3 Br + Br (CH3)3CBr Step 1 Slow Br (CH3)3C H (CH3)3C H2O Step 2 Fast H O H (CH3)3COH2+ O Step 3 Fast (CH3)3COH H+ The key is the relative rate of the steps. + Br (CH3)3CBr Step 1 Slow Br (CH3)3C The slow step determines the rate of the reaction. It is unimolecular. Rate = - d [(CH3)3CBr] = k [(CH3)3CBr] dt The reaction is called an: SN1 reaction S for substitution N for nucleophilic 1 for unimolecular Br CH3 Br (CH3)3CBr Why does t-butylbromide react via an SN1 reaction while methylbromide reacts via an SN2 reaction? Size Matters Steric Hindrance HO Why does t-butylbromide react via an SN1 reaction while methylbromide reacts via an SN2 reaction? 1. Steric Hindrance 2. More stable carbocation CH3 H versus CH3 CH3 H H Transition State Intermediate Br Transition State H O H Ea (CH3)3C Br + H2O (CH3)3C OH2 + Br- Transition States occur at a maximum of a Energy Profile. T.S. T.S. Int. Intermediates occur at a minimum of an Energy Profile. They are potentially isolatable species.







