Dr. Becich's Presentation - Association for Pathology Informatics
advertisement

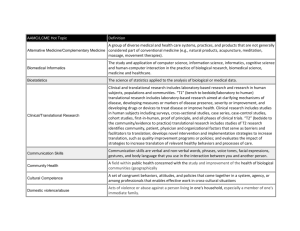
Training in Pathology Informatics Funding Opportunities Michael J. Becich, MD PhD Course Director, APIII (http://apiii.upmc.edu) Chairman, Dept of Biomedical Informatics Professor of Biomedical Informatics, Pathology, Information Science and Telecommunications (http://www.dbmi.pitt.edu) Center for Clinical and Translational Informatics (http://www.ctsi.pitt.edu/content.asp?id=1435) Overview • NCRR - CTSA Clinical Research Training Programs • NLM - Biomedical Informatics Training Programs • Quantitative Mentored Investigator Awards • K99/R00 • CAP Foundation and Industry Funding • Conclusions National Center for Research Resources Accelerating research from basic discovery to improved patient care community engagement animal model resources science education Pre-clinical Community Improved patient care imaging & informatics advances research capacity & training Clinical clinical research support NIH Roadmap – http://nihroadmap.nih.gov mouse MRI insertion tube http://ctsaweb.org/ NCRR Building A National CTSA Consortium 38 Sites with CTSA Awards since 2006 (Goal = 60) Funded CTSA sites First Round - 2006 • • • • • • • • • • • • Duke Clinical and Translational Science Institute Duke University Irving Institute for Clinical and Translational Research Columbia University Mayo Center for Translational Science Activities Mayo Clinic Oregon Clinical and Translational Research Institute Oregon Health and Science University Rockefeller University Center for Clinical and Translational Science The Rockefeller University The UCSF Clinical and Translational Science Institute University of California, San Francisco UC Davis Clinical and Translational Science Center University of California, Davis University of Pennsylvania Institute for Translational Medicine and Therapeutics University of Pennsylvania University of Pittsburgh Clinical and Translational Science Institute University of Pittsburgh University of Rochester Clinical and Translational Sciences Institute University of Rochester School of Medicine and Dentistry University of Texas Houston Center for Clinical and Translational Sciences University of Texas Health Sciences Center at Houston Yale Center for Clinical Investigation Yale University Funded CTSA sites Second Round - 2007 • • • • • • • • • • • • Atlanta Clinical and Translational Science Institute (Atlanta-CTSI) Emory University (partnering with Morehouse School of Medicine) CTSA at Case Western University Case Western University CTSA at Weill Cornell Medical College Weill Cornell Medical College (partnering with Hunter College) Johns Hopkins Institute for Clinical and Translational Research Johns Hopkins University Michigan Institute of Clinical and Health Research University Of Michigan At Ann Arbor North and Central Texas Clinical and Translational Science Initiative University of Texas Southwestern Medical Center – Dallas University Of Wisconsin Madison Institute for Clinical and Translational Research University Of Wisconsin Madison University of Chicago CTSA University Of Chicago University of Iowa's Institute for Clinical and Translational Science University Of Iowa University of Washington Institute of Translational Health Sciences University Of Washington Vanderbilt Institute for Clinical and Translational Research Vanderbilt University (partnering with Meharry Medical College) Washington University Institute of Clinical and Translational Sciences Washington University Funded CTSA sites Third Round - 2008 • • • • • • • • • • • • • • Albert Einstein-Montefiore Institute for Clinical and Translational Research Albert Einstein College of Medicine (partnering with Montefiore Medical Center) Clinical and Translational Science (BU-BRIDGE) Institute Boston University Colorado Clinical and Translational Sciences Institute University of Colorado Denver Harvard Catalyst: The Harvard Clinical and Translational Science Center Harvard University Indiana Clinical and Translational Science Institute Indiana University School of Medicine Institute for Integration of Medicine and Science (IIMS) The University of Texas Health Science Center at San Antonio Northwestern University Clinical and Translational Sciences Institute Northwestern University The Ohio State University Center for Clinical and Translational Science The Ohio State University The Scripps Translational Science Institute The Scripps Research Institute The Stanford Center for Clinical and Translational Education and Research Stanford University Translational and Clinical Science (TraCS) Institute The University of North Carolina at Chapel Hill Tufts Clinical and Translational Science Institute Tufts University UAB Center for Clinical and Translational Science The University of Alabama at Birmingham University of Utah Center for Clinical and Translational Science The University of Utah 52 Sites with CTSA Planning Grants • • • • • • • • • • • • • • • • • • • • • • • • • • Brown University Children's Research Institute Dartmouth College Emory University Georgetown University Harvard University Medical School Howard University Pennington Biomedical Research Center Medical College of Georgia Medical College of Wisconsin Medical University of South Carolina Meharry Medical College Methodist Hospital Research Institute Michigan State University Mount Sinai School of Medicine New York University School of Medicine Stanford University State University New York Temple University Texas A&M University Health Science Center Translational Genomics Research Institute University of Massachusetts Medical School University of Medicine Dentistry of New Jersey University of Alabama University of Arkansas for Medical Sciences University of Cincinnati • • • • • • • • • • • • • • • • • • • • • • • • • • University of Colorado Denver Health Sciences University of Connecticut School of Medicine University of Florida University of Hawaii University of Illinois University of Kansas Medical Center University of Kentucky University of Louisville University of Maryland School of Medicine University of Missouri University of New Mexico University of North Carolina University of North Dakota University of Oklahoma Health Sciences Center University of South Carolina University of South Florida University of Southern California University of Tennessee Health Science Center University of Texas Health Science Center University of Virginia University of Washington University of Wisconsin Virginia Commonwealth University Wake Forest University Health Sciences Wayne State University Weill Medical College Cornell University Informatics at first 12 CTSA sites MJB’s Impression (disclaimer!!!) Data Core, Ctr, Research Clinical Bioinform Site Education Warehous Inst, Dept IT Trials DB atics e Columbia Core Yes Yes Yes Duke Core Yes Mayo Core Yes Yes Yes Yes Oregon Core Yes Yes Yes Rockefeller Core Yes Yes Yes UC Davis Core Yes Velos Yes Yes UCSF Core Yes Yes Yes Yes Yes U Penn Ctr(s) x 2 Yes Yes Yes U Pitt Dept, Ctr Yes Yes U Rochester Core Yes Yes Yes Yes UTHSC Core Yes Yes Yes Yes Yale Core Yes Yes Yes Yes Yes 12 8 8 7 6 Clinical Info Web Portal Yes Yes Yes Vocab Architectu Biospeci Ontology re men Info Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes Yes 5 Yes Yes 5 4 3 • All Twelve CTSA sites have a component of Research IT support – Includes implementation, enhancement, support of existing systems • Pathology Informatics is grossly under-represented (except maybe in Tissue Banking Informatics) • Punch line – every program in CTSA (as a requirement for funding) has to have a training program in clinical research and have a biomedical informatics core!!! Yes 3 CTSA Training Programs • CTSA has (by requirement) training programs (K and T programs of NIH) and clinical and translational research centers (CTRCs, formerly GCRCs) • K Programs (for junior faculty) – • • • • Career Development and Mentorship Programs Clinical Research Scholars Program Focus is to take MDs and train them in clinical research Pathology is not taking advantage of this major opportunity • T Programs (for residents and fellows) • Clinical Research Training Program – Certificate program (1 yr) available at most institutions • Designed for trainees – clinical & translational research fellowship • Pathology is not taking advantage of this major opportunity Total number of active trainees 160 140 120 Non-degree Students Residents CRSP (K12) CETP CRTP 100 80 60 40 20 0 2000 2001 2001 2003 2004 2005 2006 NLM Biomedical Informatics Training Programs = 20 http://www.nlm.nih.gov/ep/GrantTrainInstitute.html NLM Biomedical Informatics Training Programs = 18 • 1. University of California Irvine Irvine, CA, Program contact: Pierre Baldi • 2. University of California Los Angeles Los Angeles, CA, Program contact: Alex Bui • 3. Stanford University Stanford, CA, Program contact: Russ Altman • 4. University of Colorado Denver/ Aurora, CO, Program contact: Larry Hunter • 5. Yale University New Haven, CT, Program contact: Sandra Frawley • 6. Regenstrief/Indiana University Indianapolis, IN, Program contact: Steve Downs • 7. Harvard University Boston, MA, Program contact: Lucila Ohno-Machado • 8. Johns Hopkins University Baltimore, MD, Program contact: Kersti Winny • 9. University of Missouri-Columbia Columbia, MO, Program contact: Bill Caldwell • 10. Columbia University Health Sciences NY, NY Program contact: Marina Honablue • 11. Oregon Health & Science University Portland, OR; Program contact: Bill Hersh • 12. University of Pittsburgh Pittsburgh, PA; Program contact: Toni Porterfield • 13. Vanderbilt University Nashville, TN; Program contact: Cindy Gadd • 14. Rice University Houston, TX; Program contact: Tony Gorry • 15. University of Utah Salt Lake City, UT; Program contact: Scott Narus • 16. University of Virginia Charlottesville, VA; Program contact: Stephanie Guerlain • 17. University of Washington Seattle, WA; Program contact: Brian Brown • 18. University of Wisconsin Madison Madison, WI; Program contact: Louise Pape NOTE: Red #s are sites that do not yet have a CTSA Funded Training Program NLM Training Programs • Focused on 3 types of training: – PhD – major focus – MS – major focus – Certificates (one year generally) – excellent for Pathology Informatics trainees • Great for informatics networking even if your program is “stand alone” • Short term training opportunities also available – summer, 3 month rotation • Major demand see 10x10 program – http://www.amia.org/10x10 Quantitative Mentored Investigator Award (K25) http://grants.nih.gov/grants/guide/pa-files/PA-06-087.html The K25 award will provide support and “protected time” for a period of supervised study and research for productive professionals with quantitative (e.g., mathematics, statistics, economics, computer science, imaging science, informatics, physics, chemistry) and engineering backgrounds to integrate their expertise with NIH-relevant research. Requires mentor Requires training plan Requires research plan K99/R00 (so called “kangaroo”) Pathway to Independence Awards Purpose The NIH Pathway to Independence Award will provide up to five years of support consisting of two phases. The initial phase will provide 1-2 years of mentored support for highly promising, postdoctoral research scientists. This phase will be followed by up to 3 years of independent support contingent on securing an independent research position. The PI Award is limited to postdoctoral trainees. This program expires January 3, 2010 unless reissued. NLM supports research career development in biomedical informatics and bioinformatics. We define informatics as the intersection of computer, information and behavioral sciences with one or more application domains. Application domains of interest include health care delivery, basic biomedical research, clinical and translational research, public health and others. Whatever the application domain, the research career focus must be informatics. Preference will be given to applicants who received their informatics training at one of NLM's university-based training programs in Biomedical Informatics. NLM Contact Clinical and Public Health Informatics: Dr. Hua-Chuan Sim, simh@mail.nih.gov Bioinformatics and Computational Biology: Dr. Jane Ye, yej@mail.nih.gov Deadlines for New Applications: February 12, June 12, and October 12 each year Deadlines for Revised Applications: March 12, July 12, and November 12 each year Full listing of deadlines for competing applications: http://www.nlm.nih.gov/ep/Deadlines.html CAP Foundation Scholars Research Program – sponsored by CAP and Seracon Award Amount: Up to $12,500 for six months Up to $25,000 for one year • • • • • • Intended for salary support. Recipient’s institution is expected to contribute benefits and overhead costs and may provide a support supplement. Assurance must be given that facilities and direct support sufficient for the work proposed by the applicant will be provided throughout the term of the grant. This award is made to the scholar, not the institution. It may, with cause, be terminated at any time. Recipients who choose not to accept the award may list the recognition on their curriculum vitae. Purpose: The purpose of the award is to help facilitate the transition from residency to an independent investigator. Therefore, persons who have already been awarded major research support for the previous academic year will not be eligible. Time Commitment: Projects must be completed within one (1) year. For those who are in a second year fellowship, the project must be completed within three (3) years after completing residency. Applicants must plan to spend their fellowship year in basic or applied research, and are expected to work full-time as defined in their project plan. Eligibility: Residents at the PGY 2 level, and up to those doing a second year fellowship. Residents who are more than three years post-residency training are not eligible. Applicants must be a CAP Member or have an application pending. http://www.cap.org/apps/cap.portal?_nfpb=true&_pageLabel=foundation CAP Foundation Advanced Training in Pathology Informatics - Sponsored by McKesson • Award Amount: Allowance provided for travel and living expenses. It is expected that the recipient’s own institution will continue to provide for salary and benefits during the award period. • Purpose: To offer background and experience to pathology residents to introduce and implement advanced informatics technology in the clinical laboratory. After the rotation, the resident will be able to: • Identify the following factors important in introducing and utilizing new information technology in the clinical laboratory: – – – – – – • • • Medical decision support systems and artificial intelligence Electronic medical record Digital multimedia and imaging applications Telepathology and telemedicine Outcomes research and data mining Process control, quality control, and quality assurance Demonstrate ability to recognize and utilize information concerning new trends and technology in pathology. Commitment: Four (4) weeks, must be completed between 5/1/09 – 8/31/09. Eligibility: Pathology residents or those in fellowships. It is a requirement that candidates have adequate training and experience in informatics. Applicants must be a CAP Junior Member. CAP Foundation Research in Telepathology - Sponsored by Nikon • Award Amount: $5,000 to cover expenses incurred during the grant period, and for travel to attend a national meeting to present an abstract/paper summarizing study results. • Purpose: To further developments in and use of information technology for the practice of pathology. • Time Commitment: This grant is intended to facilitate a sixmonth study. • Eligibility: Pathology residents and those in fellowships who are still in training. Applicants must be a CAP Junior Member or have an application pending. CAP Foundation Research in Telepathology - Sponsored by Olympus • Award Amount: $5,000 to cover expenses incurred during the grant period, and for travel to attend a national meeting to present an abstract/paper summarizing study results. • Purpose: To further developments in and use of information technology for the practice of pathology. • Time Commitment: This grant is intended to facilitate a sixmonth study. • Eligibility: Pathology residents and those in fellowships who are still in training. Applicants must be a CAP Junior Member or have an application pending. Conclusions • Pathology Informatics trainees should consider clinical research training programs in CTSA sites – 38 • Pathology Informatics trainees should consider biomedical informatics training programs – 18 • Other funding opportunities exist in your institution, foundations and from industry – Communicate with CAP Foundation • Make it happen, there are options!!! End of Talk – e-mail me at becich@pitt.edu if you have questions or need clarifications about the discussion. NOTE: Please e-mail me if you want a copy of this presentation or contact me for additional help in training resources nationally – 412-623-3941