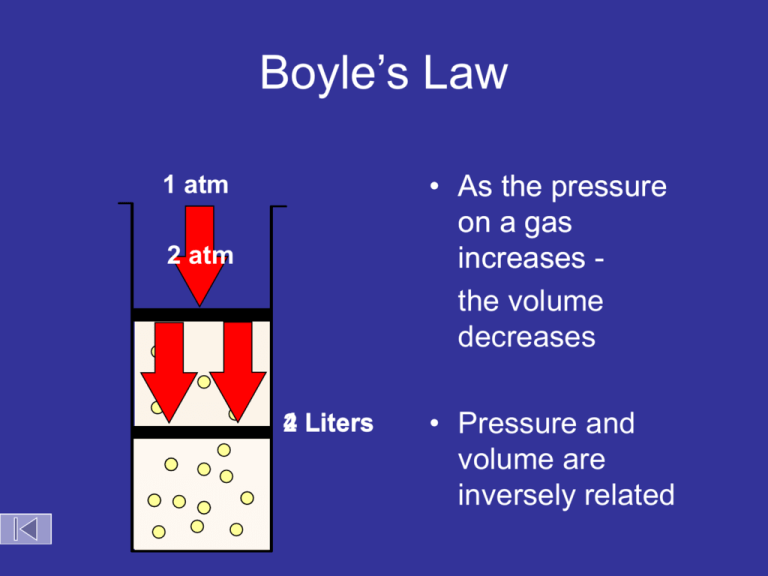
Boyle’s Law
• As the pressure
on a gas
increases the volume
decreases
1 atm
2 atm
4 Liters
2
• Pressure and
volume are
inversely related
Boyle’s Law
Timberlake, Chemistry 7th Edition, page 253
Boyle’s Law
P1V1 = P2V2
(Temperature is held constant)
Timberlake, Chemistry 7th Edition, page 253
P vs. V (Boyle’s law)
At constant temperature and
amount of gas, pressure
decreases as volume increases
(and vice versa).
P1V1 = P2V2
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Digital
Text
Copyright © 2007 Pearson Benjamin Cummings. All rights reserved.
Boyle's Law
If n and T are constant, then
PV = (nRT) = k
This means, for example, that
Pressure goes up as Volume goes down.
A bicycle pump is a good example of Boyle's law.
As the volume of the air trapped in the pump is
reduced, its pressure goes up, and air is forced
into the tire.
Robert Boyle
(1627 - 1691)
Son of Early of Cork, Ireland.
• As the pressure
on a gas
increases the volume
decreases
1 atm
2 atm
4 Liters
2
• Pressure and
volume are
inversely related
• As the pressure
on a gas
increases the volume
decreases
2 atm
2 Liters
• Pressure and
volume are
inversely related
Boyle’s Law Data
Pressure-Volume Relationship
250
(P3,V3)
Pressure (kPa)
200
150
(P1,V1)
100
(P2,V2)
50
P1 = 100 kPa
V1 = 1.0 L
P2 = 50 kPa
V2 = 2.0 L
P3 = 200 kPa
V3 = 0.5 L
P1 x V1 = P2 x V2 = P3 x V3 = 100 L x kPa
0
0.5
1.0
1.5
Volume (L)
2.0
2.5
P vs. V (Boyle’s Data)
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 404
Pressure vs. Volume
for a Fixed Amount of Gas
(Constant Temperature)
Pressure
(Kpa)
100
150
200
250
300
350
400
450
600
Volume (mL)
500
400
300
Volume
(mL)
500
333
250
200
166
143
125
110
PV
50,000
49,950
50,000
50,000
49,800
50,500
50,000
49,500
200
100
0
100
200
300
Pressure (KPa)
400
500
Pressure vs. Reciprocal of Volume
for a Fixed Amount of Gas
(Constant Temperature)
0.010
1 / Volume (1/L)
0.008
Pressure
(Kpa)
100
150
200
250
300
350
400
450
0.006
0.004
0.002
0
100
200
300
Pressure (KPa)
400
Volume
(mL)
500
333
250
200
166
143
125
110
500
1/V
0.002
0.003
0.004
0.005
0.006
0.007
0.008
0.009
Boyle’s Law Illustrated
Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 404
Boyle’s Law
Volume
The
Pressure
P.V
pressure
and volume
(torr)
(mL.torr)
of 10.0
a gas are 760.0
inversely7.60 x 103
related
20.0
379.6
7.59 x 103
(mL)
•at constant253.2
mass & temp
30.0
7.60 x 103
40.0
191.0
7.64 x 103
P
PV = k
V
Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem
Pressure and Volume of a Gas
Boyle’s Law
A quantity of gas under a pressure of 106.6 kPa has a volume
of 380 dm3. What is the volume of the gas at standard
pressure, if the temperature is held constant?
P1 x V1 = P2 x V2
(106.6 kPa) x (380 dm3) = (103.3 kPa) x (V2)
V2 = 400 dm3
PV Calculation (Boyle’s Law)
A quantity of gas has a volume of 120 dm3 when confined
under a pressure of 93.3 kPa at a temperature of 20 oC.
At what pressure will the volume of the gas be 30 dm3 at
20 oC?
P1 x V1 = P2 x V2
(93.3 kPa) x (120 dm3) = (P2) x (30 dm3)
P2 = 373.2 kPa
Volume and Pressure
Two-liter flask
One-liter flask
The molecules are
closer together; the
density is doubled.
Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 101
The average molecules hits the wall
twice as often. The total number of
impacts with the wall is doubled and
the pressure is doubled.
Volume and Pressure
Two-liter flask
One-liter flask
The molecules are
closer together; the
density is doubled.
Bailar, Jr, Moeller, Kleinberg, Guss, Castellion, Metz, Chemistry, 1984, page 101
The average molecules hits the wall
twice as often. The total number of
impacts with the wall is doubled and
the pressure is doubled.








