ACTIVITY II-ORGANIC CHEMISTRY ALL CELLS MAKE A HUGE
advertisement

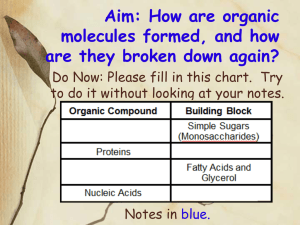
ACTIVITY II-ORGANIC CHEMISTRY ALL CELLS MAKE A HUGE NUMBER OF LARGE MOLECULES FROM A SMALL SET OF SMALL MOLECULES THAT ENTER CELLS FROM OUTSIDE THE CELLS PART A: TEACHER DEMO (toys) TEACHER DEMO: TAKE NOTES BELOW: 1. What are monomers? 2. What are polymers? 3. What are macromolecules? 4. Is there directionality to building polymers? 5. What is dehydration synthesis (condensation reaction)? 6. What is hydrolysis? STUDENT NOTES: DISCUSSION-MARZANO STYLE 1 PART B: DEHYDRATION SYNTHESIS ACTIVITY-BUILDING A CARBOHYDRATE MATERIAL: blue paper and other colored paper, scissors, tape, glucose paper model, and water paper model 1. Copy the glucose molecule onto a variety of colored papers and copy the water drops onto blue paper. 2. Take 2 differently colored glucose molecules and number the carbons as shown below. 3. Join (bond) the two glucoses together by cutting off an –H– from carbon number 1 of one molecule and an –OH– from carbon number 4 of another glucose molecule. 4. Tape the 2 molecules together forming a glycosidic bond. 5. Take the trimmed H-O-H, affix it to the water drop, and tape the water drop onto the glycosidic bond. 6. Once this is complete you will a disaccharide (two simple sugars linked together). 7. Take you disaccharide and bond it in the same manner with another pair of students using the same paper dehydration synthesis process. 8. Continue with additional groups until you have a multi-colored long chain polysaccharide (many simple sugars linked together) which can be hung on the walls of the classroom. 2 PART C: WEB ANIMATION VIEW THE ANIMATION BELOW WHILE ANSWERING THE QUESTIONS: DEHYDRATION SYNTHESISCAND HYDROLYSIS (http://www.phschool.com/science/biology_place/biocoach/bioprop/monomers.h tml) ANSWER THE FOLLOWING QUESTIONS: 1. What sizes are most biomolecules? 2. How are macromolecules or polymers built? 3. What process links monomers together to make a polymer? 4. What is removed from each monomer before linking? 5. What is required to catalyze (speed up) this reaction? 6. Is the catalyst changed in the reaction? 7. What process breaks apart a polymer into its monomers? 8. What is required to catalyze (speed up) this reaction? 9. Is the catalyst changed in the reaction? DISCUSSION: PART D: READING Most of the organic compounds we will study are examples of polymers. A polymer is a large chemical compound (macromolecule) composed of smaller repeating units --- over & over & over again. Like a long choo-choo train is made up of smaller connected, repeating, choo-choo cars. Each individual choo-choo car is a monomer. Thus, monomers are the smaller molecules that are linked together to make the polymer (bigger molecule-entire choo-choo train). The chemical process that connects the smaller subunits to form large organic compounds is called dehydration synthesis. "Synthesis" means to build. The "dehydration" part of the term refers to the fact that water is lost during the chemical process that bonds the subunits together. We will "see" this in a minute when we get more specific. Hydrolysis is the process that breaks large organic compounds into their smaller subunits. It is the opposite of dehydration synthesis. In HYDROlysis, water (hydro) is added and the large compounds are split ("lysis" means split). The process of hydrolysis is involved in digestion --- when food is broken down into nutrients. 3 REVIEW: COMPLETE CHART PROCESS STARTS WITH ... Small molecules (subunits) Water & large molecule ENDS WITH ... EXAMPLE X If a polymer made up of sixteen monomers was completely broken apart into sixteen separate monomers in the process of dehydration synthesis, How many water molecules will be formed? CLUE SHEET: 4