Intermolecular Forces, Liquids, Solids Chemistry Presentation
advertisement

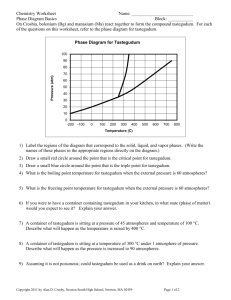
Chapter 13 INTERMOLECULAR FORCES, LIQUIDS, AND SOLIDS 13.0 Objectives Use the kinetic molecular theory to compare the three states of matter at the molecular level. Describe and give examples of the types of intermolecular forces: dipole-dipole interactions, hydrogen bonds, and dispersion forces. Explain the unusual properties of water with reference to hydrogen bonding. Define and explain properties of liquids; equilibrium vapor pressure, boiling point, critical temperature, and critical pressure. Describe the bonding and forces inside different types of solids. Sketch and identify various landmarks on a phase diagram; phase boundaries, critical temperature and pressure, and triple point. Homework HW#1 - 17, 19, 21, 23, 51, 53 HW#2 - 25, 27, 31, 33, 35 Liquids HW#3 - 55, 57, 59, 71 Intermolecular Forces “Little bit of everything” HW#4 - 45, 47, 49 Phase Changes 13.1 STATES OF MATTER AND THE KINETIC MOLECULAR THEORY 1. Particles in the solid, liquid and gas states 1. Gas – “molecules far apart” 2. Liquid – “molecules closer together” 3. Solid – “molecules extremely close together” 13.1 STATES OF MATTER AND THE KINETIC MOLECULAR THEORY 2. Internal forces: intermolecular vs. intramolecular forces a. Definitions Intermolecular: forces between molecules; molecules interact with one another Intramolecular: forces within the molecule; hold the atoms together (bonds) 13.1 STATES OF MATTER AND THE KINETIC MOLECULAR THEORY b. importance. Combination of intermolecular and intramolecular forces largely explains chemical properties 13.2 INTERMOLECULAR FORCES 1. Dispersion forces, London forces, van der Waals forces, induced-dipole/induceddipole forces (all these terms are synonymous) a. definition and mechanism force of attraction between induced-dipoles Form from distortion of electron clouds when molecules approach each other ALL molecules have some dispersion forces acting between them Only force of attraction in liquids and solids of nonpolar molecules 13.2 INTERMOLECULAR FORCES b. Trends As the molecular mass increases, the dispersion forces increase; more opportunity for distortion About 1/10 the strength of a dipole-dipole force Occur in both nonpolar and polar molecules Weakest of the intermolecular forces 13.2 INTERMOLECULAR FORCES 2. Dipole/dipole forces a. definition Permanent dipoles that try to align with + and - ends of adjacent molecules; polar molecules Induce some dispersion forces in addition to the permanent + and - ends Mid-range of the forces 13.2 INTERMOLECULAR FORCES 13.2 INTERMOLECULAR FORCES a. trends Higher BP and MP than their similar molar mass but nonpolar counter parts because stronger forces from both dipole-dipole and dispersion forces (stronger intermolecular forces more resistance to change phase) 13.2 INTERMOLECULAR FORCES 3. Dipole/Induced-dipole forces Dipoles cause nonpolar molecules to polarize slightly, thus forming temporary dipoles Polarization – the process of inducing a dipole In general the greater the molar mass the more polarizable a molecule (difficult to measure exp) 13.3 HYDROGEN BONDING 1.Definition and mechanism Force in which a hydrogen atom covalently bonded to a nonmetal is simultaneously attracted to a neighboring nonmetal atom Very large dipole in molecules Nonmetal should be small, very electronegative Hydrogen bonding is FON! Usually occurs only with F, O, and N 2.Examples H2O, NH3, HF 13.3 HYDROGEN BONDING 3. Unusual properties of water Ice forms rigid, open structures Molecules with higher M.M. have lower BP than water Increases volume upon freezing (floats) H2O vs. CH4 Surface Tension and Capillary Action 13.3 HYDROGEN BONDING 13.4 SUMMARY OF INTERMOLECULAR FORCES 1. Relative strengths of intermolecular forces Dispersion < Dipole-Dipole < Hydrogen Bonding (Dipole/induced-dipole falls b/t disp. And d-d) All molecules will have dispersion Combination of these forces explain much BP, MP, surface tension, solubility 13.4 SUMMARY OF INTERMOLECULAR FORCES 2. Ex13.1 Arrange the following in order of increasing melting point/boiling point: HCl, F2, I2, HF, H2O, CH4, CH3Cl, CH3NH2, CH2O 13.5 PROPERTIES OF LIQUIDS A. Vapor Pressure 1. Dynamic equilibrium – characteristics “Two opposing processes occur at the same rate” Evaporation Condensation No process wins out (net of zero once equilibrium is reached) 13.5 PROPERTIES OF LIQUIDS 2. Liquid-vapor equilibrium Molecules of a liquid evaporate (convert from liquidgas) in a closed vessel until equilibrium is reached between evaporation and condensation Hand Sanitizer Scenario: 1. Liquid has molecules with Avg. K.E. (temp) 2. Some molecules at surface have a little extra K.E., break intermolecular attractions become gas 3. Molecules leave, thus taking some of the K.E. from liquid (temp. falls!) 13.5 PROPERTIES OF LIQUIDS 13.5 PROPERTIES OF LIQUIDS 3. Equilibrium vapor pressure A.k.a “Vapor Pressure” Pressure exerted by a vapor once equilibrium is established 13.5 PROPERTIES OF LIQUIDS 4. Ex13.2 Carbon disulfide (CS2) has a vapor pressure of 298mmHg at 20.0oC. A sample of 6.00g of CS2 is put into a stoppered flask at that temperature. A. What is the maximum volume of the flask if there is to be a liquid-vapor equilibrium? B. If the flask has a volume of 3.00L, what will be the pressure of the carbon disulfide vapor? C. If the flask has a volume of 6.00L, what will the pressure of the vapor be? 13.5 PROPERTIES OF LIQUIDS B. Vapor Pressure and Temperature 1. See graph Pg.13 in Reference Booklet Movie Clip: Heating Curve and V.P. 13.5 PROPERTIES OF LIQUIDS 2. Clausius-Clapeyron Equation Method for experimentally determining the Hvap A plot of Vapor Pressure vs. 1/T (in K) is a straight line with a slope of –Hvap/R 13.5 PROPERTIES OF LIQUIDS 3. Ex13.3 Carbon disulfide has a vapor pressure of 41.4mmHg at -22.0oC and 100.mmHg at -5.00oC. Estimate the heat of vaporization of carbon disulfide, and the vapor pressure of CS2 at 28.0oC. 13.5 PROPERTIES OF LIQUIDS C. Boiling Point 1. See graph Pg.13 in Reference Booklet Temperature at which the vapor pressure equals prevailing atmospheric pressure 2. Normal boiling point, Standard boiling point Boiling point at 1 atm of pressure 13.5 PROPERTIES OF LIQUIDS 3. Vacuum pump demo 4. Pressure cooker Operates on a similar principle…but opposite to the vaccuum pump demo 13.5 PROPERTIES OF LIQUIDS D. Critical Temperature and Pressure 1. Definitions Critical point – the point where the interface between liquid and vapor disappears Critical temperature-temp at the critical point Critical pressure-pressure at the critical point Super critical fluid-state at and above critical point that has a liquid like density and vapor like flow 13.5 PROPERTIES OF LIQUIDS 2. Ex13.4 The critical point of CO is -139oC, 35.0atm. Liquid CO has a vapor pressure of 6.00atm at -171oC. Which of the following statements is true? A. B. C. D. CO is a gas at -171oC and 1.00atm. A tank of CO at 20oC can have a pressure of 35.0atm. CO gas cooled to -145oC and 40.0atm will condense. The normal boiling point of CO is above -171oC. 13.6 SOLID-STATE CHEMISTRY: METALS 1. Particles and Force of Attraction 2. Properties Cations that have delocalized e- Conduct e- and heat Luster Malleable, ductile Hardness varies from soft to hard 3. Examples Copper, Brass, Steel 13.7 SOLID-STATE CHEMISTRY: STRUCTURES AND FORMULAS OF IONIC SOLIDS 1. Particles and forces of attraction 2. Properties Formed by “electrostatic interactions” between cations and anions (lattice energy); Repeating Structure Hard, brittle, high MP, soluble in H2O, conduct as liquids 3. Examples NaCl, CaI2, LiBr 13.8 OTHER KINDS OF SOLID MATERIALS A. Network Covalent solids 1. Particles and forces of attraction 2. Properties Network of covalent bonds that extends throughout a crystalline structure Giant molecule of many repeating units Very hard, high M.P., generally nonconductors 3. Examples Diamond: hard, does not conduct Graphite: formed into sheets, used as lubricant, conducts 13.8 OTHER KINDS OF SOLID MATERIALS 13.8 OTHER KINDS OF SOLID MATERIALS B. Molecular solids 1. Particles and forces of attraction 2. Properties Molecules are held together in a crystal structure by intermolecular forces (all types) Low to moderate MP and BP; soft; poor conductivity 3. Examples H2O, C6H12O6, CO2 13.8 OTHER KINDS OF SOLID MATERIALS C. Amorphous Solids 1. Particles and forces of attraction 2. Properties Covalent networks with no specific arrangements; irregular Shape can be changed somewhat easily Noncrystalline; wide range of MP; poor conductivity 3. Examples Glass, Polyethylene, Nylon 13.9 THE PHYSICAL PROPERTIES OF SOLIDS 1. Continuum of strengths of bonds Ionic > Metallic > Network Cov > Covalent > H-Bonding > Dipole-Dipole > Dispersion 13.10 PHASE DIAGRAMS Graph that shows what phase of matter (solid, liquid, gas) a substance will be in under conditions of Temp and Pressure 13.10 PHASE DIAGRAMS 1. Know the location of the following on a phase diagram: gas, liquid, and solid areas, triple point, critical T and P, normal bp, relative densities of solid and liquid. See Diagrams. 13.10 PHASE DIAGRAMS 2. Ex13.6 A pure substance has a vapor pressures of 320.mmHg at 125oC, 800.mmHg at 150.oC, and 60.0mmHg at the triple point, 85.0oC. The melting point of the substance increases slightly as pressure increases. Sketch the phase diagram for this substance. Estimate the normal boiling point of the substance. What changes occur as the temperature drops from 150.0oC to 100.0oC at a constant pressure of 320.mmHg? 13.10 PHASE DIAGRAMS Phase diagram of CO2