Intermolecular Forces
advertisement

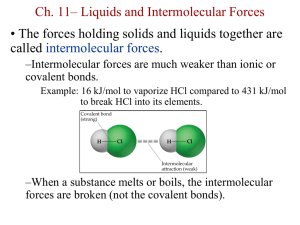
Intermolecular Forces and Bonding in Solids 1 Different States of Matter 3 States Solid state - ice Liquid state - water Gas state - water vapor 2 Intermolecular Forces Intermolecular forces are forces between molecules. Intramolecular forces hold atoms together in a molecule. Intermolecular vs Intramolecular • 41 kJ to vaporize 1 mole of water (inter) • 930 kJ to break all O-H bonds in 1 mole of water (intra) “Measure” of intermolecular force Generally, intermolecular forces are much weaker than intramolecular forces. boiling point melting point DHvap DHfus DHsub 3 Intermolecular Forces Dipole-Dipole Forces: Attractive and repulsive forces between polar molecules solid liquid 4 Intermolecular Forces Hydrogen Bond: a special dipole-dipole interaction between the hydrogen atom in a polar N-H, O-H, or F-H bond and an electronegative O, N, or F atom. A H…B or A H…A A & B are N, O, or F 5 Intermolecular Forces Dispersion Forces: Attractive forces that arise as a result of temporary dipoles induced in atoms or molecules 6 Dispersion Forces among Nonpolar Molecules separated Cl2 molecules instantaneous dipoles 7 Intermolecular Forces Polarizability is the ease with which the electron distribution in the atom or molecule can be distorted. Polarizability increases with: • greater number of electrons • more diffuse electron cloud Dispersion forces usually increase with molar mass. 8 Intermolecular Forces What type(s) of intermolecular forces exist between each of the following molecules? CH4 CH4 is nonpolar: dispersion forces. S SO2 SO2 is a polar molecule: dipole-dipole forces. There are also dispersion forces between SO2 molecules. HF HF is a polar molecule: dipole-dipole forces. Hydrogen is bounded to F. Hydrogen bonds exist. There are also 9 dispersion forces between HBr molecules. Structures and Types of Solids A crystalline solid possesses rigid and long-range order. In a crystalline solid, atoms, molecules or ions occupy specific (predictable) positions. An amorphous solid does not possess a well-defined arrangement and long-range molecular order. A unit cell is the basic repeating structural unit of a crystalline solid. lattice point Unit Cell Unit cells in 3 dimensions 10 Seven Types of Unit Cells 11 Three Types of Cubic Cells 1 atom/unit cell 2 atoms/unit cell 4 atoms/unit cell (8 x 1/8 = 1) (8 x 1/8 + 1 = 2) (8 x 1/8 + 6 x 1/2 = 4) 12 13 The Striking Beauty of Crystalline Solids 14 Three Types of Crystalline Solids atomic solid ionic solid molecular solid 15 Types and Properties of Solids 16 Metallic Crystals • • • • Lattice points occupied by metal atoms Held together by metallic bonds Soft to hard, low to high melting point Good conductors of heat and electricity nucleus & inner shell e- mobile “sea” of e17 MO Energy Levels as a Function of the # of AO 18 The band of MOs in lithium metal Conduction Band Valence Band 19 Covalent Crystals • • • • Lattice points occupied by atoms Held together by covalent bonds Hard, high melting point Poor conductor of heat and electricity carbon atoms diamond graphite 20 Graphite Consists of Layers of Carbon Atoms 21 Electrical Conductivity in Graphite sp2 hybridization Delocalized p orbitals conductor 22 Electrical Conductivity in Diamond sp3 hybridization localized s orbitals insulator 23 Conductor, Semiconductor, and Insulator conductor insulator semiconductor 24 Doped Semiconductors n-type p-type 25 Chemistry In Action: High-Temperature Superconductors 26 Acknowledgment Some images, animation, and material have been taken from the following sources: Chemistry, Zumdahl, Steven S.; Zumdahl, Susan A.; Houghton Mifflin Co., 6th Ed., 2003; supplements for the instructor General Chemistry: The Essential Concepts, Chang, Raymon; McGraw-Hill Co. Inc., 4th Ed., 2005; supplements for the instructor Principles of General Chemistry, Silberberg, Martin; McGraw-Hill Co. Inc., 1st Ed., 2006; supplements for the instructor 27