TPG 4140 Natural Gas
advertisement

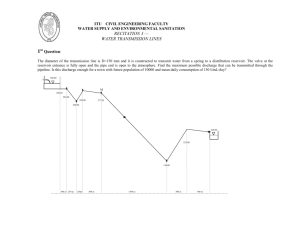
TPG 4140 Natural Gas SUGGESTED SOLUTIONS Examination, December 20, 2013 Professor Jon Steinar Gudmundsson PROBLEM 1 Average result 72 % (a) We need to find the density of the natural gas at 500 kPa pM zRT All the parameters are known except the molecular weight. We have M = 28.97 such that M = 0.65∙28.97 = 18.8 kg/kmol 500 103 18.8 3.98 kg / m3 0.9857 8314 (273.15 15) We need to find the mass flow rate m = ρ∙u∙A The flow area is A d 2 4 (0.11)2 4 0.00950 m2 m = 3.98∙20∙0.00950 = 0.756 kg/s = 2720 kg/h The volumetric flow rate at 500 kPa is q500 = m/ρ = 0.756/3.98 = 0.190 m3/s = 684 m3/h Converting to standard conditions we use p Ts.c. 1 qs.c. q p s.c. T z 1 500 288.15 1 3 qs.c. 684 3425 Sm / h 101.3 288.15 0.9857 The capacity in terms of kW we calculate from qkW = 36 MJ/kg ∙ 0.756 kg/s = 27.2 MW = 27,200 kW (b) We need to determine the Reynolds number Re Re ud 3 0.11 3.98 119,620 0.01098 103 Because the Reynolds number is close enough to 100,000 we can use the Blasius equation f 0.316 Re 0.25 f 0.316 0.0170 1196200.25 It would also be OK to use the Haaland-equation, but then by setting the relative roughness, k/d, equal to zero. If we need the approximate pressure drop we can choose to use the Darcy-Weisbach equation. Start by using inlet density. p f f L 2 u 2 d We can start by using the density of the natural gas at 500 kPa p 0.0170 1600 3.98 32 4430 Pa 4.4 kPa 0.044 bar 2 0.11 We note that the pressure drop of 4 kPa is small (ca. 0.8 %) compared to the pipeline pressure at inlet of 500 kPa so the density (at inlet) used above is reasonable. Note also that the pressure drop is given as bar, not bara or barg. Several students forgot to write the viscosity with 10-3. And, surprisingly, the pipe diameter in Reynolds number was assumed to be the pipe length, 1600 m. Also, several students did not write 3x3 (velocity squared) in the Darcy-Weisbach equation. 2 A more comprehensive pressure drop calculation would be to use d A2 M d p 22 2 2 p p ln L 0 2 1 f p12 f m 2 z RT where the natural logarithm part can be assumed small compared to other parts. The resulting pressure drop would be the same. One of the lessons here is that by looking carefully at the problem, one is able to use the simplest equations. Knowing the theory and practice well, can make most calculations simple. PROBLEM 2 Average result 86 % (a) US Henry Hub has been lower than UK NBP. (b) Coal. 3 (c) Methane, ethane, propane and butane. Gudmundsson, October 3, 2012. 4 (d) Groningen natural gas contains a lot of nitrogen. (e) LNG, LPG and condensate. (f) Russia, Norway and Algeria. 5 (g) Buyer pays for agreed volume, even though not all the volume is needed (used). (h) Spherical tanks and membrane tanks. PROBLEM 3 Average result 88 % (a) 6 The key to solving this problem is to find the gas density at standard conditions, remembering that the z-factor equals unity (=1). And, remembering that the pressure in atmosphere must be multiplied by 105 to convert to Pa. M stands for 106. pM zRT 1.013 105 20 kg 0.846 3 1 8314 (15 273.15) m m q 1 106 0.846 1 kg 9.8 24 3600 s (b) The key to solving this problem is to know the Hammerschmidt equation, and to use the enclosed table with the constant K and molecular weight M of the antifreeze MEG T K xmass M 1 xmass K 1 1222 1 0.985 M T 62 20 1 x 1 0.985 1 x x x 1 0.5 1.985 (c) The key to solving this problem is to know the Wobbe equation and to know how the gas gravity can be obtained from the molecular weight. M = 28.97 kg/kmol Gas gravity 20/28.97=0.69 WI 40 40 MJ 48.2 3 Sm 0.69 0.83 PROBLEM 4 Average result 85 % 7 (a) Base-load, peak shaving and small-scale. (b) Corrodes aluminium (heat exchangers). (c) Required power increases with temperature. (d) Column B. PROBLEM 5 Average result 90 % (a) We use the equation for temperature drop in a pipeline U d T2 T T1 T exp L m C p T2 = 20 C T=5C T1 = 80 C 20 5 0.2 80 5 ln(0.2) = -1.61 24 inch = 24∙2.54 cm = 0.61 m Ud 15 0.61 0.117 10 3 mC p 70 3500 1.61 = 0.117∙10-3∙L L = 13.8 km 8 Because 9 km < 13.8 km, natural gas hydrate will not form in the bulk flow of the pipeline at steady-state flow conditions. However, because the pipeline wall temperature is lower than the bulk temperature, some hydrate may form at the pipeline wall (this fact needs not to be elaborated in the answer). Another way to solve the problem is to calculate the outlet temperature directly T2 5 80 5 exp 0.117 10 3 9000 5 75 0.349 31 C Because 31 > 20 will natural gas hydrate not form in the bulk of the flow from the template to the platform. (b) At long-term shut-in of the pipeline at normal operating pressure, the pipeline temperature will gradually approach the seawater temperature of 5 C. Because 5 C < 20 C, natural gas hydrate will form in the pipeline. Long-term is not specified in the problem but should be understood to be long enough for the whole pipeline to cool down. (c) The most common method used to prevent the formation of natural gas hydrate in a pipeline is to inject antifreeze at the pipeline inlet. The most common antifreeze used is MEG (mono ethylene glycol). Methanol is also used in some pipelines. A new method to prevent the formation of natural gas hydrate is to insulate and/or heat the pipeline, by either hot oil in a bundle or electrical heating. If hydrate forms at steady-state flow conditions, MEG needs to be injected continuously (or the pipeline heated continuously). If hydrate forms only at long-term shut-in, MEG needs to be injected before the pipeline is shut-in (or the heating oil or electrical heating turned on). The worst case scenario is if the pipeline is shut-in in an emergency situation and no MEG has been injected. The pipeline must be put on-line as quickly as possible; that is, before it cools down below 20 C. PROBLEM 6 Average resultat 15 % To give a good answer here it is necessary to have a good overall knowledge of what has been presented throughout the course. The figures below are taken from the presentation about the Aasta Hansteen field development. (a) 9 A high inlet pressure is required to make the gas flow from the field to the receiving facility/terminal. The whole pipeline must be able to withstand the inlet pressure; for example in case of sudden shut-in. It is typical that the inlet pressure is about 200 bara and the outlet pressure 100 bara. (b) The 60 C limit is likely to be determined by the outlet temperature from the export compressor, and the thermal expansion/contraction due to start-up and shut-in. The -10 C limit is likely based on the possibility that the gas can expand in a JouleThomson manner (J-T effect), with significant cooling. The sea temperature is around 5 C. (c) The upper limit must be lower than the design pressure. The lower limit is likely based on the need for the gas to be above the cricondenbar; that is, to avoid condensation of liquid hydrocarbons. (d) The inlet temperature depends on the compression (compression increases temperature) on the Spar platform. The temperature profile depends on the exponential of distance (see cooling of pipelines with distance). (e) The Aasta Hansteen platform is a platform in a new area with several prospects nearby. The pipeline is designed to accommodate natural gas for fields that will be developed in the near future. The pipeline capacity must therefore be higher than the gas production rate from AH. 10 11