Alcohols - mquagliaoths
advertisement

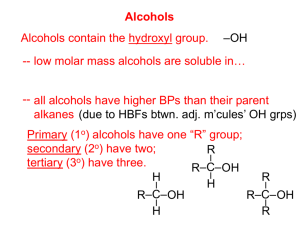
SCH 4U Name: _______________________ Unit: Organic Chemistry Date: _______________________ An Introduction to Alcohols The simplest way to think of alcohols is as a carbon chain attached to one of the spots on a water molecule where a hydrogen atom is usually located. A hydrogen atom will be removed from water so the –OH, now called a hydroxyl group, can attach to a carbon chain. Usually this occurs across a double or a triple bond and so is classified as an addition reaction. This type of addition reaction is further classified as hydration because it involves the addition of a water molecule. Note that the hydroxyl group is a hydrogen atom covalently bonded to an oxygen atom which is then covalently bonded to a carbon chain. The hydroxyl group is thus entirely different from the polyatomic ion, the hydroxide ion, which carries with it a net negative charge and forms ionic bonds with positively charged ions…like this… Ionic bond [Na]1+ [OH]1- This is the hydroxide ion. It is a polyatomic ion with a net charge and forms ionic compounds. The addition of the OH group (the hydroxyl group) will drastically change the properties of the related hydrocarbon. This is because the O-H bond is so polar. The O-H bond will allow for hydrogen bonding to occur between neighboring alcohol molecules and between alcohol molecules and water so the boiling point of an alcohol is much higher than that of the corresponding hydrocarbon and the alcohol is also much more water soluble. Example: ethane (hydrocarbon) ethanol (alcohol) δ+ δHydrogen bonding in addition to London forces δ+ Only weak London forces between neighboring molecules. δ- Non-polar molecule Boiling temperature – 89oC Insoluble in water Polar molecule Boiling temperature +78oC Very soluble in water Nomenclature of Alcohols In the IUPAC naming system, the OH functional group adds the suffix –ol to the ending of the typical chain. The parent alkane is the longest chain that contains that OH group. Example: CH3OH methanol this is a highly toxic alcohol that leads to blindness and often death CH3CH2OH ethanol used in alcoholic beverages, lacquers, varnishes and perfumes CH3CH2CH2OH 1-propanol or propan-1-ol CH3CH(OH)CH3 2-propanol or propan-2-ol solvent for lacquers and waxes and in brake fluid common name is isopropanol; sold as rubbing alcohol; used in the manufacture of gums, oils and acetone More than one naming system is in current use for alcohols. Use the method that makes the most sense to you. OH Newer system Older name, still in use. Your book uses it. Classification of Alcohols 1. Primary, Secondary and Tertiary Alcohols Alcohols are categorized based on the type of carbon that they are attached to. Since carbon atoms have 4 bonds, the atom that has the OH can be attached to 1,2 or 3 carbon atoms as well. This creates primary, secondary and tertiary alcohols respectively. The classification is important for the types of reactions that each will undergo because the products are determined by the availability of H atoms in key positions. Examples: 3- methylbutan-1-ol is a primary (1o) alcohol The carbon attached to the hydroxyl group is attached to only one other carbon. Pentan-2-ol is a secondary (2o) alcohol 2-methylbutan-2-ol is a tertiary (3o) alcohol You draw the structural formula here… The carbon attached to the hydroxyl group is attached to two other carbons. 2) Polyalcohols Alcohols that contain more than one OH group are considered to be diols or triols. Antifreeeze for example is ethane-1.2-diol (or 1,2-ethanediol), more commonly known as ethylene glycol. A common triol is propane-1,2,3-triol (or 1,2,3-propanetriol) which is known as glycerol. Glycerol is non-toxic. It is a common ingredient in skin moisturizers, lipsticks and chocolate. glycerol 3) Cyclic alcohols These are less common, but the naming procedure is consistent with our system to this point. The following are some examples: OH OH Cyclohexanol OH OH phenol 1,2-dihydroxybenzene Practice 1) Write IUPAC names for the following molecules. It might be an idea to draw out a structural formula for each one before trying to name it. a) CH3CH(OH)CH2CH3 b) CH3CH(OH)CH2CH2CH2(OH) c) C6H5(OH)2 2) Draw a structural formula for each of the following: a) 3-methylbutan-1-ol (could be called 3-methyl – 1 – butanol) b) propane-1,2 – diol (could be called 1, 2 – propanediol) c) propane-1,3-diol (could be called 1, 3 – propanediol) d) pentane – 1, 3, 4 – triol (could be called 1,3,4-pentanetriol) e) cyclopentanol f) 2-methylphenol g) 2-methylcyclohexanol h) all the structural isomers of C5H11OH 3) Decide whether each of the following compounds is a primary, a secondary or a tertiary alcohol. b) a) c) d) 3) Answer #2 on page 27 of the textbook. 4) Write out a balanced chemical equation to show the production of the most common alcohol product from the addition reaction between pent-2-ene and water. Use structural formulas in your balanced chemical equation. Name the product of the reaction. (Remember to use Markovnikov’s Rule, page 26.)