Regents Unit 15b: Halides, Alcohols, & Ethers
advertisement

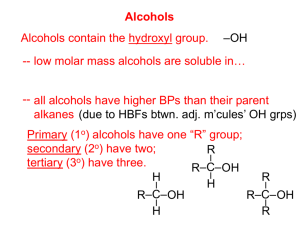
Hydrocarbon Derivatives: Halocarbons, Alcohols, & Ethers Hydrocarbons • Contain only carbon & hydrogen • But carbon can form strong covalent bonds to other elements, incl. O, N, F, Cl, Br, I, S, & P Functional Group • One or more H’s in a hydrocarbon can be replaced by an atom or group of atoms. • An atom or group of atoms in an organic molecule that always behaves in the same way is called a functional group. • Adding a functional group changes the chemical & physical properties in specific ways, depending on the functional group. Intermolecular Forces • Determine Boiling Point & Solubility • Van der Waals or dispersion – nonpolar – weakest. • Dipole-dipole – intermediate. Molecule must have atoms with different electronegativities & not arranged symmetrically. • Hydrogen bonding – strongest. Molecules must contain H bonded to F, O, or N. Functional Groups • • • • • • • • Halocarbons Alcohols Ethers Amines Aldehydes Ketones Carboxylic Acid Ester • Amide • Amino Acid Organic Halides • One or more of the hydrogen atoms in an alkane is replaced with a halogen (F, Cl, Br, or I). • Not hydrocarbons! Often called halocarbons or alkyl halides. Naming Halides • Figure out the base name. • Use prefixes to specify substituent: fluoro, chloro, bromo, iodo • If more than one, use di, tri, etc. to specify # of substituents. • If necessary, give locations of halogens by numbering C-atoms in backbone. CH3Cl CH3CHFCH3 H H–C–Cl H H H H H–C–C–C–H H F H chloromethane 2-fluoropropane C 3H 7F Naming Halides CH3CCl2CHClCH3 H Cl H H H–C–C–C–C–H H Cl Cl H 2,2,3-trichlorobutane C4H7Cl3 Ranking Halogens • If more than 1 kind of halogen atom, list them alphabetically. • Chain is numbered to give lowest number to halogen that comes first in alphabet. Different Halogens 4 3 2 1 Chlorine is 1st alphabetically, so it determines numbering. 2-chloro-4-fluoro-3-iodobutane Name: Br CH3CH2CHCHCH3 I 3-bromo 2-iodo pentane F Cl HCCH F Cl 1,1-dichloro-2,2-difluoroethane Properties of Halocarbons • For an alkane & an alkyl halide of similar size & shape, the alkyl halide has the higher boiling point & higher density. Why? Stronger intermolecular forces. • CH4: bp = -162C & density = 0.423 g/ml • CH3Cl: bp = -24C & density = 0.911 g/ml What intermolecular forces for CCl4? CH3CH2CH2CH2CH3 pentane CH3CH2CH2CH2CH2F 1-fluoropentane CH3CH2CH2CH2CH2Cl 1-chloropentane CH3CH2CH2CH2CH2Br 1-bromopentane CH3CH2CH2CH2CH2I 1-iodopentane Boiling Point (C) 36 Density (g/ml) 0.626 63 0.791 Inc 108 0.882 130 1.218 155 1.516 Inc Uses of Alkyl Halides • Cleaners & solvents • Teflon & PVC’s are alkyl halides. • Refrigerants. (used to be chlorofluorocarbons. Now hydrofluorocarbons.) • Starting materials in many reactions. Halogen Derivatives • • • • • • CH3Cl = local anesthetic CHCl3 = solvent, general anesthetic CHI3 = antiseptic CCl4 = dry cleaning solvent CF2Cl2 = refrigerant Fluorocarbons = teflon, lubricants, synthetic blood • Chlorofluorocarbons = aerosol propellants, refrigerants Table R • General Formula for halocarbons: R-X • R represents the entire hydrocarbon part of the molecule. (The alkyl part.) • X represents the halogen (F, Cl, Br, or I). Alcohols • An –OH group replaces a H in a hydrocarbon. • The –OH group is called the hydroxyl group. H HCH H -OH H HCOH H Alcohols are nonelectrolytes! • The hydroxyl group resembles the hydroxide ion of inorganic bases, but it does not form ions in water! • The hydroxyl group is polar. So alcohols are soluble in water. Naming AlcohOLs • Based on alkane name. • Name the parent chain. – Drop the –e and add –OL. • If the parent chain has 3 or more C atoms, number the C’s & give the location of the – OH group. Naming H H H H HCCCCH OH H H H 1-Butanol bp = 100C H H H H HCCCCH H OH H H 2-Butanol Bp = 115C Note: Never more than 1 –OH group per C More than 1 hydroxyl group • Prefixes di-, tri-, tetra- used before the –ol ending to tell the # of hydroxyl groups. • So don’t drop the -e from the alkane name. • These hydroxyls are on different C atoms! Classifying Alcohols • By # of hydroxyl groups –Monohydroxy: 1 hydroxyl group –Dihydroxy: 2 “ “ –Trihydroxy: 3 “ “ • By position of each hydroxyl group on main carbon chain. Monohydroxy Alcohols • Primary: hydroxyl group attached to end C of chain or branch. • Secondary: hydroxyl group attached to C in a chain – C is bonded to 2 other C’s. • Tertiary: hydroxyl group attached to C at a branch point – C is bonded to 3 other C’s. HHHH H-C-C-C-C-O-H HHHH HHHH H-C-C-C-C-H HHOH H 1-butanol primary 2-butanol secondary H H-C-H H H H-C-C-C-H HOH H 2-methyl 2-propanol Tertiary HH H-C-C-H OO HH Dihydroxy HHH H-C-C-C-H OOO HHH Trihydroxy Properties of Alcohols • Contain a H bonded to an O atom. • Therefore Hydrogen Bonding occurs. • Alcohols have a higher boiling point than the corresponding alkane. • Like dissolves Like. Alcohols tend to be very soluble in water. R R - O H + O H + - Which compound has the highest boiling point? A. B. C. D. CH4 C 2H 6 C 3H 8 C3H7OH Correct response = D. Table R • General Formula for Alcohols: ROH. • R represents the entire hydrocarbon part of the molecule. • OH is the hydroxyl group. Ethers • General formula is ROR where R may or may not be the same as R. • R and R are hydrocarbon branches. • O is an oxygen bridge. • Ethers are not linear. They are bent, like water. Properties of Ethers • In a pure ether, no hydrogen bonding – no H bonded to O. Do have weak dipoledipole interactions – bent, like H2O. • Dipole-dipole interactions are between dispersion forces & hydrogen bonding. • Ethyl ether once used as anesthesia Properties of Ethers • Compared to alkanes: – Higher boiling pts than similar alkanes. – More soluble in water than alkanes. • Compared to alcohols: – Lower boiling pts than similar alcohols. – Much less soluble in water than alcohols. Naming Ethers • If the 2 hydrocarbon branches are identical, name the branch (once) & add the word ether. • If the 2 branches are different, list them in alphabetical order followed by the word ether. H H HCOCH H H Methyl Ether H H H H H H HCCCOCCCH H H H H H H Propyl Ether H H H H HCOCCCH H H H H Methylpropyl Ether