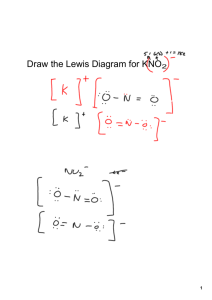
Ion Exchange Resin - Environmental Water Systems
advertisement

The Basics of Demineralisation by Ion Exchange Raw Water Supply Water comes into sites from many sources and can be potable (suitable for drinking), an industrial supply provided by the local water plc or the clients own supply extracted on site from a river / borehole. The drinking water use is sterile and includes all many of the natural elements we need to sustain a healthy life. All these ions however cannot be left in the water fed to boilers and to other processes. They would cause corrosion and deposits affecting performance and causing premature plant failure. Raw Water Supply Water in the UK can come from many sources: Four principle types of supply are widely encountered 1. 2. 3. 4. Ground Waters – Pumped from boreholes or wells, these supplies have a high salts content. From deep boreholes the water quality remains very constant and it is normally high in hardness (calcium & magnesium) and high in alkalinity (bicarbonate). Normally dissolved organics are not present. Surface Waters – Upland sources low in dissolved solids but with a high proportion of dissolved organics. Surface Waters – Lower levels with moderate dissolved solids and with moderate to high organics. Mix of surface and ground waters of variable quality – (river supplements) Raw Water Supply Ions present in all natural waters: Cations Sodium (Na) Calcium (Ca) Magnesium (Mg) Potassium (K) Iron (Fe) Anions Bicarbonate (HCO3) / Carbon Dioxide (CO2) Sulphate (SO4) Chloride (Cl) Nitrate (NO3) Silica (SiO2) In addition dissolved organics can be present which can be important on some sites with regard to resin selection. Typical IEx Plant Designs To achieve high water quality the majority of plants employed in the UK fit into the following categories: 1. 2. 3. 4. Cation – Anion (Main subject for today’s presentation) Cation – Anion – Polishing Cation Cation – Anion – Mixed Bed Reverse Osmosis – Ion Exchange Plant The cation and anion columns can employ either co-flow or counter-flow regeneration and in some cases they can also they employ a Degassing Tower after the cation unit. Ion Exchange Resin - Properties Synthetic Ion Exchangers require certain properties to perform demineralisation. The three main properties required are: a. Insoluble in, but permeable by water. b. An ability to exchange ions, with the different types of ions commonly encountered in water supplies. Active groups throughout the beads perform the ion exchange. c. To allow the passage of water through the resin bed at optimum rates without undue pressure drop. Cation Exchange Resins Two principle types of cation resin: Weak Acid Cation – with carboxylic group (Resin – COOH) – Dealkalisation Process Strong Acid Cation – with sulphonic acid group (Resin - S03H) Regeneration is with an excess amount of dilute acid (sulphuric or hydrochloric acid). Cation Unit Representation in service and after co-flow regeneration Raw Water In Service OperationResin Resin - SO3H + Na Resin - SO3Na + H 2Resin - SO3H + Ca 2Resin – SO3Ca + 2H Calcium Magnesium Sodium H+ (unused) In Service Order of Selectivity: Fe > Ca > Mg > K > Na In Regeneration – (Typically with 5% HCl conc.) Resin – SO3Na + HCl Resin – SO3H + NaCl + Excess Acid Resin – SO3Ca + H2SO4 Resin – SO3H + CaSO4 + Excess Acid Treated water contains high concentration of H+ ions so water exit cation has a low pH. Anion Resins Strong base anion resins are employed on all demineralisation plants for producing high quality water. Either in separate anion units and or as the strong base anion component in mixed beds. Strong base anion resins will remove all anions present but require an excess of Sodium Hydroxide (Caustic Soda) to regenerate them. Anion Unit Representation in service and after co-flow regeneration Raw Water In Service OperationResin Sulphate Nitrate Chloride Bicarbonate / CO2 Silica OH- (unused) In Service Resin – Amine OH + Cl Resin – Amine Cl + OH 2Resin – Amine OH + SO4 2Resin – Amine SO4 + 2OH Order of selectivity: SO4 > NO3 > Cl > Bicarbonate / CO2 > Silica In Regeneration (Typically with 4% NaOH conc.) Resin – Amine Cl + NaOH Resin – Amine OH + NaCl + Excess NaOH Resin – Amine SO4 + NaOH Resin – Amine + Na2SO4 + Excess NaOH Treated water now contains OH- ions which combine with H+ ions to form pure water H2O. Ion Exchange Resin Standard grade resins from all manufacturers are typically made 300 to 1200 microns with less than 1% less than 300 microns. Hence internal systems / nozzles are selected to have a maximum slot / aperture of 200 microns. In addition resin suppliers also make more uniform and specialist grades. Ion Exchange Resin Grades Narrow Uniform Grade Resins Most Narrow grade resins typically in the range of 400 – 800 microns (some of these resins have a very narrow distribution and a low uniformity coefficient ) Standard grade resins 300 – 1200 microns. Narrow grade resins can offer: a. Higher capacity b. Better Rinse c. Lower pressure drop d. Higher breaking weight e. Are more suitable to some specialist engineering designs (e.g. Packed beds) Ion Exchange Resin Selection The Six Most Important Factors Affecting Resin Selection: Raw water quality. (TDS and other contamiants) Treated water quality. (conductivity / silica specification) Engineering techniques employed. (co-flow or counter flow regen) Operating flow rate. (good kinetics) Process temperatures. (anion resins have low maximum temp limits) Presence of organic foulants. (anion resin resistant to fouling) Degassing Towers Between the cation and anion stage on many large demin plants there is a degassing tower. (Normally if the bicarbonate content of the raw water supply is above 50 mg/l). These are a very efficient way of removing the bicarbonate present in the water mechanically and cheaply. When the Ca / Mg associated with the bicarbonate passes through a cation resin this happens. Ca(HCO3)2 + Resin-2H+ Resin-Ca + H2CO3 (Carbonic acid) When the resin releases the H+ ions the water becomes acidic (pH 2-3 exit SAC). At low pH Carbonic acid is unstable. H2CO3 at low pH H2O + CO2. (forming pure water and carbon dioxide) Co-flow vs Counter Flow Regeneration (Cation Representation) Service flow Counter flow (Example showing upflow regen.) Co-flow Co-flow Regeneration After regen: Counter Flow Regeneration After regen: Ca Ca Ca Ca Mg Mg Mg Mg Na Na Na Na Na Na Na Na With counter flow regeneration the most highly regenerated portion of the ion exchange bed is at the unit outlet so leakage is significantly better in service operation! Co – Flow Regeneration The regeneration of the resin involves the following main steps with co-flow regeneration Backwash Bed Settle Establish motive water Regenerant Injection Slow / Displacement Rinse Fast Rinse Co-Flow Regeneration 7 1 Feed Water Valve Identifiers 5 1. Inlet 2. Oulet 3. Drain 4. Regen / Slow Rinse Inlet 4 6 5. WWI 6. WWO Regen 7. Vent (manual) Effluent 3 2 Treated Water Plant Operation / Treated Water Quality SAC / Degasser / SBA / Mixed Bed Treated water Quality Cation TWQ: Anion TWQ: MB TWQ: pH 2 – 3 pH > 7 AT ALL TIMES!!!!! Conductivity Increase (R water x 1.5 to 2) Conductivity low pH 7+ (Depending Sodium leakage exit cation) Conductivity 0.056 - 0.1 us/cm Reactive Silica low Na < 0.01 mg/l Co-flow Regen (Typ.) Co-flow Regen (Typ.) Silica < 10 - 20 ug/l 0.5-2.0 mg/l Na 0.05 – 0.3 mg/l SiO2 Counter flow Regen (Typ.) Counter flow Regen (Typ.) Trace Na / No hardness 0.02-0.5 mg/l Na SAC 5 mg/l CO2 Degasser Typically 7.3 - 9 0.025 – 0.1 mg/l SiO2 SBA Mixed Bed Minimum Level of Instrumentation for Cation – Anion – Polishing M Bed (Cation – Anion with co-flow regeneration) Pump Raw Water Flow Pressure Cation Pressure Anion Pressure Conductivity Silica (Optional depending on clients Treated Water specification) Treated Water Minimum Level of Instrumentation for Cation – Anion – Polishing M Bed (Cation – Anion with co-flow regeneration) Flow Flow Pressure Pressure Pump Cation Anion LS Pressure Pressure LS Raw Water Tank Degasser Tower Conductivity Silica (Optional depending on clients Treated Water specification) Pump Treated Water Tank