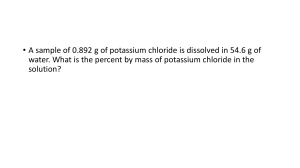Molarity and Molality Chemistry Presentation
advertisement

CP Chemistry Ms. Morrison MOLARITY AND MOLALITY NOTES Concentration definition: the measure of how much solute is dissolved in a specific amount of solvent can be dilute to concentrated can use specific numerical quantities to measure concentration molarity molality Molarity concentration expressed as the number of moles of solute per liter of solution use M, molar ex. 2.5 M solution is a 2.5 molar solution Conversion reminder: to convert mL to L, must divide by 1000 (1000 mL = 1 L) Molarity Equations 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 = 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 = 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝐿𝑖𝑡𝑒𝑟𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 # 𝑜𝑓 𝑔𝑟𝑎𝑚𝑠 𝑠𝑜𝑙𝑢𝑡𝑒 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑠𝑜𝑙𝑢𝑡𝑒 𝐿𝑖𝑡𝑒𝑟𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑖𝑜𝑛 Molarity Example Problem #1 What is the molarity of a solution containing 158 grams of sodium chloride, NaCl, in 2.5 liters of water? Molarity Example Problem #2 Calculate the molarity of 250 mL solution containing 75 grams of H2SO4. Molarity Example Problem #3 How many grams of NaCl would be dissolved in 1.75 L of a 0.85 M solution of NaCl? Molality concentration expressed as the number of moles of solute dissolved in 1 kilogram of solvent volume can change with temperature but mass does not so molarity can change if temperature changes but not molality use m, Molal ex. 1.3 m solution is a 1.3 molal solution Conversion reminder: to convert g to kg must divide by 1000 (1000 g = 1 kg) Molality Equations 𝑀𝑜𝑙𝑎𝑙𝑖𝑡𝑦 = 𝑀𝑜𝑙𝑎𝑙𝑖𝑡𝑦 = 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑠𝑜𝑙𝑢𝑡𝑒 𝑘𝑔 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 # 𝑜𝑓 𝑔𝑟𝑎𝑚𝑠 𝑠𝑜𝑙𝑢𝑡𝑒 𝑀𝑜𝑙𝑎𝑟 𝑚𝑎𝑠𝑠 𝑠𝑜𝑙𝑢𝑡𝑒 𝑘𝑔 𝑜𝑓 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 Molality Example Problem #1 Calculate the molality of a solution that contains 15.7 g of NaCl in 100 g of water. Molality Example Problem #2 Calculate the molality of a solution that contains 20.0 g CaCl2 in 700 g of H2O.