Chemical Formula - EighthGradeScience
advertisement

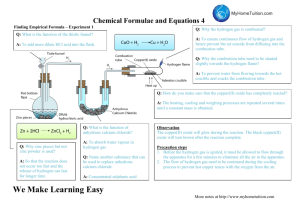
Chemical Formula matter atom molecule element O O2 compound ex: H20, NaCl symbol - p 20 •usually 1 or 2 letters that are used to identify an element •only 1st letter is capitalized (ex: He helium) symbol hydrogen H Co CO Co - cobalt CO - carbon, oxygen formula - p 20 • a combination of symbols that shows the ratio of elements in a compound • Ex: H2O, the ratio of Hydrogen to Oxygen is 2:1 formula Example (add to notes on formula) • CH4 (methane) • 1:4 ratio • 1 Carbon atom: 4 Hydrogen atoms Word Equations A word equation gives the names of the substances involved in a reaction. For example: copper + oxygen → copper oxide Copper and oxygen are the reactants, and copper oxide is the product. Chemical Equations Chemical equations give the symbols and molecular formula of the substances involved in a reaction. In the example below, if we just replace the words shown below with the correct chemical formula, we will get an unbalanced equation, as shown here: copper + oxide yields copper oxide Cu + O2 → CuO Chemical equations give the symbols and molecular formula of the substances involved in a reaction. In the example below, if we just replace the words with the correct chemical formula, we will get an unbalanced equation, as shown here: Copper + oxygen yields copper oxide Cu + O2 → CuO To make things equal, we need to adjust the number of units of some of the substances until we get equal numbers of each type of atom on both sides of the arrow. Here is the balanced chemical equation: 2Cu + O2 → 2CuO 2Cu + O2 2CuO atoms two Elements compounds fewer larger three three 10 21 more than a billion b c e a d 2 2 sulfur 1 oxygen 2 nitrogen hydrogen 1 3 3 4 3 3 3 3 2 hydrogen carbon oxygen carbon hydrogen oxygen magnesium 2 1 3 12 22 11 1 1 sulfur oxygen sodium oxygen hydrogen hydrogen 4 1 6 45 6 3 1 1 2 oxygen 2 4 2 4 iron oxygen sodium hydrogen carbon oxygen 2 5 3 1 1 1 3 6