blog 7-3. Acid base
advertisement

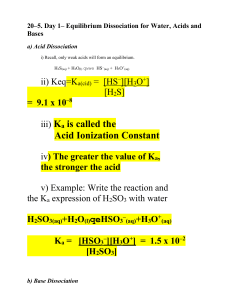
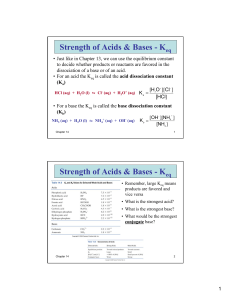
• 7-3 Acid and Base • Monoprotic acid: • an acid that donates one H+ ion per molecule. • HBr → H+ + Br - • Diprotic acid: an acid that donates two H+ ion per molecule • H2SO4 + H2 O → H3O+ + HSO4• • HSO4- + H2O → H3O+ + SO42- • Polyprotic acid: an acid that donates three or more H+ ion per molecule. H3PO4 + H2O → H3O+ + H2PO4- H2PO4- + H2O → H3O+ + HPO4 2HPO4 2- + H2O → H3O+ + PO4 3- • Polyprotic can yield a higher concentration of hydronium ions than their given concentration. H3PO4 (aq) + 3 H2O(l) PO43- (aq) + 3H3O+ (aq) 0.100 M 0.100 M 0.300 M Strength of Acids and Bases • Strength is defined in terms of the degree of dissociation of the acid or base into ions. For example: • HX + H2O • BOH → → H3O+ + X- B+ + OH- • A strong acid or base is defined as one which completely or nearly completely dissociates into ions in solution. • A weak acid or base is defined as one which only a small amount dissociates into ions in solution. • Keq = [H3O+ ][X- ] [HX] • strong acid: a very high Keq (favours the product). • weak acid: a very low Keq (favours the reactant). • Note: The equilibrium expression for an acid is referred to as a Ka; the constant for a base is a Kb. • Note: A polyprotic acid would have a different Ka for each step; the value of the Ka would decrease for each step. Strength vs. Concentration: • Strength is a measure of the degree of dissociation of an acid or base. • Concentration is a measure how many moles of acid or base are present per litre of solution. Comparing Strength of Acids: • The strength of an acid is measured by its Ka value. The higher the Ka, the stronger the acid. • If two acids are put in solution the strongest one will dominate; it will act as the acid, forcing the weaker acid to act as a base. For example: • Oxalic acid and Citric acid • HOOCCOOH • H 3C 6 H 5O 7 H+ + HOOCCOOH + + H 2C 6H 5O 7 - • Because oxalic acid is higher on the table it is the strongest acid; it will go in the forward direction and will force citric acid to act as the base and go in the reverse direction. • HOOCCOOH H+ + HOOCCOO- • H+ + H2C6H5O7 H3C6 H5O7 ___________________________________________ HOOCCOOH + H2C6H5O7- H3C6 H5O7 +HOOCCOOacid base acid base Ionization of Water and Kw • Acids and bases act in aqueous solution. There is a relationship between the hydronium ion and the hydroxide ion in solution. • The equation for the dissociation of water is: H2O(l) → H3O+ (aq) + OH- (aq) • The equilibrium constant for the dissociation of water is Keq = [H3 O+ ][OH- ] • The Keq for water, like that of acids and bases has a special notation; Kw. • At most temperatures the Kw of water is 1.00 x 10-14 Kw = [H3O+ ][OH- ] Kw = 1.00 x 10-14 • In a neutral solution the concentration of both hydronium and hydroxide ions is 1.00 x 10-7 M • If the hydronium ion concentration is greater than 1.00 x 10-7 M the solution is acidic; • If it is less than that value, the solution will be basic (since the concentration of hydronium ions is less than 1.00 x 10-7 M, the hydroxide ion concentration will be greater than 1.00 x 10-7 M).