Document
advertisement

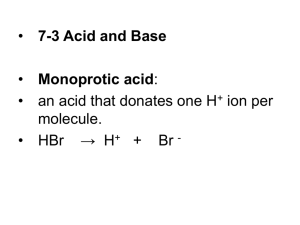
GET READY FOR YOUR QUIZ! • You need • • • • A periodic table Solubility rules A calculator A pen/pencil • When you’re done • Put your quiz in the basket • Bring me your book problems from the weekend • Tonight’s homework • Notes on section 4.3 • Read through the slides (I will give them to you when you show me your homework) • If you HAVEN’T taken 1st year chemistry, reaction balancing and classification • If you have and want a refresher, it is an optional assignment • Finish your copper oxide lab report WARM-UP • Please have your notes and practice problems out for me to check. • Write a molecular equation for the gas-evolution reaction that occurs when you mix aqueous hydrobromic acid and aqueous potassium sulfite. • Write a net ionic reaction for the reaction that occurs when you mix hydriodic acid with calcium sulfide. TYPES OF AQUEOUS REACTIONS • Precipitation reactions (you should be an expert on these now!!) • Acid-Base reactions • Gas-Evolution Reactions ACID BASE REACTIONS • An acid reacts with a base to form water and an ionic salt • Remember that salt is a generic term in chemistry – not just NaCl! • Acid provides H+ • Base provides OH• Example: nitric acid reacts with lithium hydroxide • HNO3 + LiOH –> H2O + LiNO3 ACID BASE REACTIONS • Titration – a lab procedure where a substance of known concentration is reacted with another substance in a solution of unknown concentration in the presence of an indicator • Equivalence point – the point in the titration when the number of moles of OH- equals the number of moles of H+ • Indicator – a pH dependent dye that allows the chemist to identify the equivalence point by a color change POLYPROTIC ACIDS • An acid that provides more than one hydrogen ion per molecule is called polyprotic • Examples: H2SO4 (diprotic), H3PO4 • Write a balanced equation for the reaction between sulfuric acid and sodium hydroxide POLYPROTIC ACIDS • An acid that provides more than one hydrogen ion per molecule is called polyprotic • Examples: H2SO4 (diprotic), H3PO4 • Write a balanced equation for the reaction between sulfuric acid and sodium hydroxide • H2SO4 + 2NaOH –> 2H2O + Na2SO4 H+ OR H3O+? • A hydrogen ion is a single proton • In solution, H+ ions will pair up with water molecules to form H3O+ • This is called the hydronium ion • In reality, it is more accurate to write your equations using the hydronium ion • In practice, it does not matter (neither Ms. Dandridge nor the College Board cares which one you use) GAS-EVOLUTION REACTIONS • Two aqueous solutions mix to form a gaseous product • Bubbles! • Common gases you should know • H2S • H2CO3 –> H2O + CO2 • H2SO3 –> H2O + SO2 • NH4OH –> H2O + NH3 GAS-EVOLUTION REACTIONS • Write a balanced equation (including states of matter!) for the reaction between hydrochloric acid and sodium bisulfite. HOMEWORK • Copper oxide lab due tomorrow! • Anyone who HASN’T taken 1st year chemistry – basic reaction type and balancing practice is required • Also available for anyone else who wants a refresher! • Tomorrow we will practice these reaction types