UNIT IV PPT #3 - Ka and Kb
advertisement

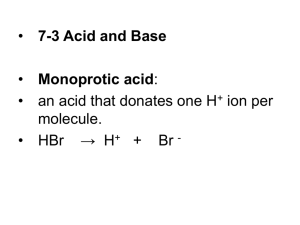
UNIT IV Ka and Kb WHAT IS KA? Recall: Find pH of 0.100 M HCl. But… What is pH of 0.10 M HF? WHAT IS KA? Look at equilibrium for weak acid HF: HF(aq) + H2O(l) H3O+(aq) + F- (aq) Keq = [H3O+][F-] [HF] For WA’s Keq is called Ka (acid ionization constant). See acid table for list of Ka’s. • higher Ka stronger acid • lower Ka weaker acid For SA’s (eg. HCl) Ka = [H3O+] [Cl-] = called “very large” [HCl] CALCULATIONS USING KA I. [H3O+] (or pH) from Ka Ex. Find the [H3O+] in 0.10 M HF. Hebden Textbook Questions #74, 75, 79, 81 CALCULATIONS USING KA II. Ka from pH Ex. A 0.350 M solution of the weak acid HA has a pH of 1.620. Find the Ka of HA. Hebden Textbook Questions #77, 80 CALCULATIONS USING KA III. Co from pH and Ka Ex. Find the concentration of HCOOH needed to form a solution with pH = 2.69. Hebden Textbook Questions #76, 78 WHAT IS KB? Base Ionization: NH3 is a very common weak base. It partially ionizes in water to form NH4+ and OH- : NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) Kb expression: Kb = [NH4+] [OH-] [NH3] Equilibrium constant is called base ionization constant (Kb). WHAT IS KB? Ex. CN- (aq) + H2O(l) HCN(aq) + OH-(aq) Kb expression: Kb = [HCN][OH-] [CN-] Ex. N2H4 (aq) + H2O (l) N2H5+ (aq) + OH-(aq) Kb expression: Kb = [N2H5+] [OH-] [N2H4] Hebden Textbook Page 128 Question #32 HOW TO DETERMINE VALUE OF KB Look at hydrolysis of base F-: F- + H2O HF + OHKb (F-) = [HF] [OH-] [F-] Look at ionization of weak acid HF: HF + H2O H3O+ + FKa (HF) = [H3O+] [F-] [HF] Multiply Ka[HF] x Kb[F-] Ka[HF] x Kb[F-] = [H3O+] [F-] [HF] x [HF] [OH-] [F-] = [H3O+] [OH-] HOW TO DETERMINE VALUE OF KB Ka(HF) x Kb(F-) = Kw Or Kb(F-) = Kw Ka(HF) In general: Kb(weak base) = Kw Ka(its conj. acid) HOW TO DETERMINE VALUE OF KB Using Acid Table: 1. 2. 3. 4. Find base on right side ( if amphiprotic -locate base on right side only). Its conjugate acid will be across from it on the left side. The Ka of its conjugate acid is on the far right of the same line. Use equation: Kb(base) = Kw Ka(conj. acid) HOW TO DETERMINE VALUE OF KB Ex. Calculate the Kb for HCO3-. Ex. Find Kb of SO32-. HOW TO DETERMINE VALUE OF KB Similarly, if Kb (base) given: Ka (weak acid) = Kw Kb(its conj. base) NOTES: • Table only states Ka values. For questions like this Kb will have to be calculated if not given. • All Ka’s on table are 2 SD’s—limits any calculation using them to 2 SD’s maximum. • The larger the Kb, the “stronger ” the weak base - the more OH- produced. • The smaller the Ka of an acid, the larger the Kb of its conjugate Base. Weaker acids have stronger conjugate bases. CALCULATIONS USING KB I. [OH-] (or pH) from Kb Ex. Find [OH-] in a 0.20 M solution of KNO2 (this is a salt, so it must be dissociated into its ions first). CALCULATIONS USING KB II. Kb from pH Ex. At a certain temperature, a 0.20 M solution of K2SO3 has a pH of 10.25. Calculate the Kb of SO32- at this temp. Hebden Textbook Page 153 Questions #84, 87, 88, 89 WHAT IS HYDROLYSIS? Reaction between a salt (ion or ions in a salt) and water to produce an acidic or basic solution. Net ionic equation for hydrolysis: ion + water molecule or ion + H3O+ or OH- SPECTATORS IN HYDROLYSIS Spectator Cations (look on Periodic Table): • Group 1 (Alkali Metal ions) eg. Li+, Na+, K+, Rb+, Cs+, Fr+ • Group 2 (Alkaline Earth ions) eg. Be+, Mg2+, Ca2+, Ba2+, Sr2+, Ra2+ Spectator Anions (look on Acid Table): • Top 5 ions on the right side of table. • ClO4- I- Br- Cl- NO3 • HSO4- is not a spectator – it is amphiprotic – will be dealt with later Spectators are eliminated in net ionic equations for hydrolysis! PROCESS FOR HYDROLYSIS Strategy: (dissociate eliminate evaluate) 1. Write dissociation equation. 2. Eliminate spectators. 3. Remaining ions: left side of table – undergo acid hydrolysis – produce H3O+ right side of table – undergo base hydrolysis – produce OH amphiprotic – determine Ka and Kb to find dominant hydrolysis HYDROLYSIS Ex. Is the salt NaF acidic, basic or neutral in water? Ex. Is the salt NH4NO3 acidic, basic or neutral in aqueous solution? Ex. Is the salt KCl acidic, basic or neutral? HYDROLYZING IONS Hydrolyzing Cations: (LEFT SIDE OF ACID TABLE) • Fe(H2O)63+ = Fe3+ • Cr(H2O)63+ = Cr3+ undergo acid hydrolysis • Al(H2O)63+ = Al3+ • NH4+ Hydrolyzing Anions: (RIGHT SIDE OF ACID TABLE) • Most of the anions from IO3- down to PO43- will undergo base hydrolysis. (Amphiprotic anions will be discussed next.) HYDROLYZING IONS Ex. Is the salt ammonium nitrite NH4NO2 acidic, basic or neutral? Ex. Determine whether the salt NH4CN (ammonium cyanide) is acidic, basic or neutral. HYDROLYZING IONS If Then the salt is: Ka (cation) > Kb (anion) Acidic Kb (anion) > Ka (cation) Basic Ka (cation) = Kb (anion) Neutral HYDROLYZING IONS Amphiprotic Anions: Ions which start with “H” and have a negative charge. Eg. HSO4- , HSO3-, H2PO4-, HPO42-, HS If Then the predominant hydrolysis is: Ka (the ion) > Kb (the ion) ACID HYDROLYSIS And, in aqueous solution, the ion: Kb (the ion) > Ka (the ion) BASE HYDROLYSIS Acts as a Base Acts as an Acid HYDROLYZING IONS Ex. Find the predominant hydrolysis of the hydrogen carbonate ion (HCO3-) and write the net-ionic equation for it. Hebden Textbook Page 148 Questions #69-73 HYDROLYSIS...PUTTING IT ALL TOGETHER Ex. Calculate the pH of 0.30 M Na2CO3. HYDROLYSIS...PUTTING IT ALL TOGETHER Ex. Calculate the pH of a 0.24 M solution of the salt aluminum nitrate.