Introduction to Organic and Biochemistry (CHE 124) Reading Assignment
advertisement

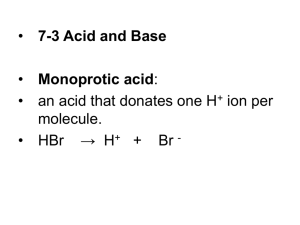
Introduction to Organic and Biochemistry (CHE 124) Reading Assignment General, Organic, and Biological Chemistry: An Integrated Approach 3rd. Ed. Ramond Chapter 7 Acids, Bases, and Equilibrium Gasses, Solutions, Colloids, and Suspensions Work Problems 7. 8, 12, 24, 26, 29, 30, 32, 36, 40, 44, 52, 56, 60, 66 Acid / Base • Acid – Sour taste (never taste lab chemicals!) – Dissolves metals – Turns litmus pink • Base – Bitter taste (never taste lab chemicals!) – Feel slippery (soapy) – Turns litmus blue • See common acids and bases Table 7.1 p. 224. Acid / Definitions • Arrhenius definition – Acid – compound that produces H+ (protons) in aqueous solution. • HCl → H+ + Cl- – Base – compound that produces OH- in aqueous solution. • NaOH → Na+ + OH- • Bronsted-Lowery definition – Acid – releases H+ (protons). – Base - H+ (proton) acceptor • HCN + H2O ⇌ CN- + H3O+ • Acid Base Base Acid arrows mean reversible • Reversible - means products can be converted into products and products can be converted into reactants. Hydrogen and Related Species • Proton (hydron) H+ • Hydronium ion (interchangable with proton) H30+ • Hydrogen atom • Hydro (hydrogen) group H· H • Hydride H: or • Hydrogen gas or molecule H:H or H¯ H2 • Hydroxide OH¯ • Hydroxyl group HO OH or Acid and Conjugate Base • Acid and conjugate base differ by presence or absence of a proton. Conjugate Acid Base HCN + H2O ⇌ CN- + H3O+ Conjugate Base Acid Amphoteric – compound that can act as an acid or a base. Equilibrium • Consider the reversible reaction of decomposition of dinitrogen tetroxide to form nitrogen dioxide. – See Fig. 7.2 p. 226 N2O4 (g) ⇌ 2 NO2(g) colorless brown Eventually the color stops changing (getting browner). This is equilibrium – the rate of the forward and reverse reaction are equal. The concentration of each species remains constant. Note the double arrow. Equilibrium Constant • If the concentration of the reactant and product of an equilibrium equation are determined then the following equation is true. • Keq = [NO2]2 = 4.6 x 10-3 [N2O2] Keq = Products Reactants [ ] = molarity Writing Equilibrium Equation Keq • To write an equilibrium constant (Keq) equation. – Before you start, BALANCE the EQUATION! – ONLY SPECIES WHOSE CONCENTRATION CAN CHANGE ARE INCLUDED. – Do NOT include solvents or solids in the equation. aA + bB ⇌ cC + dD A,and B are reactants C and D are products a,b,c,and d are coefficients Keq = [C]c [D]d [A]a [B]b What does Keq Tell us? • Keq > 1 (larger number) – [reactant] < [product] – Favors products • Keq < 1 (small number) – [reactant] > [product] – Favors reactants • Keq = 1 – [reactant] = [product] Ka • Ka = acidity constant – Special name of Keq for acid base reactions. • pKa = -log Ka. Le Chatelier’s Principle • Le Chatelier’s Principle states that when a reversible reaction is pushed out of equilibrium, the reaction responds to reestablish equilibrium. – Vary [reactant] or [product] by adding (or removing) reactants or products. Example of Le Chatelier’s Principle carbonic anhydrase H2O(l) + CO2 (g) ⇌ H2CO3(aq) water carbon dioxide carbonic acid – Describe where / why this reaction occurs? – Describe what happens if you increase [CO2] • Reaction proceeds in the forward direction (to the right) – Describe what happens if you decrease [CO2] • In which direction does the reaction proceed. – Describe what happens if you increase [H2CO3] Catalysts • Catalyst increase the rate of the reaction by lowering the activation energy. – Catalyst • Do not alter the equilibrium • Do not alter the Keq. Water is Amphoteric • Amphoteric a compound that can act as an acid or a base. HCl + H2O Acid ⇌ Cl- + H3O+ Base Base Acid NH3 + H2O ⇌ HN4+ + OHBase Acid Acid Base Water Can Ionize H2O (l) + H2O (l) ↔ H3O+ (aq) + hydronium ion Acid Base Acid OH(aq) hydroxide ion Base Kw = [H3O+][OH-] = 1 X 10-14 Kw is water equilibrium constant. pH pH = -log [H3O+] • Measure of [H3O+] • scale is continuum from 0 - 14 – 7 is neutral; • Neutral - neither acidic or basic – 0 - 6.99 is acidic – 7.01 - 14 is basic (alkaline) • one pH unit change represents 10 fold change in [H+] • See Fig. 7.6 p 233 • See Table 7.3 p. 233 pH of Strong Acids • Strong Acid – dissociates 100% in water. HCl → H+ + ClHCl HBr HI HClO4 HNO3 H2SO4 hydrochloric acid (muriatic acid) hydrobromic acid hydriodic acid perchloric acid nitric acid sulfuric acid • [H+] is equal to the [H+] of the acid • Weak Acid – dissociates less than 100% in water. – All other acids pH of Strong Bases • Strong Base – dissociate 100% in water. NaOH → Na+ + OHLiOH NaOH KOH Ca(OH)2 Sr(OH)2 Ba(OH)2 Lithium hydroxide Sodium hydroxide Potassium hydroxide Calcium hydroxide strontium hydroxide barium hydroxide • pOH is dependent on the concentration of the strong base • Weak Base – dissociates less than 100% in water. Neutralization • Neutralization reaction of an acid and base to form water and a salt. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) Acid Base Salt Water – If equal amounts of acid and base are added, the pH will equal 7. • Titration – A technique used to determine the concentration of an acid or base solutions. • Uses Buret and and an indicator • See p. 238 Fig. 7.9 pH effects the Concentration of the Acid and Conjugate Base • A few points to understand: – When pH = pKa • [acid] = [conjugate base] – When pH < pKa • [acid] > [conjugate base] – When pH > pKa • [acid] < [conjugate base] • This alters the charge on many biological molecules by changing them form the acid form to the base form (carboxylate ion) – Use the fatty acid example. Buffer • Buffer - substance that resists changes in pH thus stabilizing its relative pH. Buffers are often a solution containing a weak acid and its conjugate base – See example on next slide. – – Buffers work within 1 pH unit either side of the pKa of the weak acid. Buffer • Carbonic acid is a weak acid. It dissociates in aqueous solution to form hydronium ion and bicarbonate H2CO3 ↔ H3O+ carbonic acid hydronium ion + HCO3bicarbonate Buffering Blood • pH of blood = 7.35 – 7.45 • Blood carries many acids which can alter it’s pH. – Fatty acids, lactic acid, phosphoric acid, carbonic acid. • Body uses two approaches to control pH (p.245 Fig 7.12) – Use of Buffers (see next slide) • Carbonic Acid / Bicarbonate buffer system – Reduce [H3O+] (see following slides) • Action of lungs • Filtering by kidneys Use of Buffers • Carbonic acid is a weak acid that buffers blood. It dissociates in aqueous solution to form hydronium ion (acid) and bicarbonate. H2CO3 ↔ H 3O + carbonic acid hydronium ion + OH↔ H2O + HCO3bicarbonate + H+ Acidosis • Acidosis - low blood pH • Leads to light headedness, coma, death. – Respiratory Acidosis • Characteristics – Low blood pH; high blood PCO2; normal or high (if compensating) blood HCO3- • Causes – Diseases / conditions that limit carbon dioxide exchange by lungs such as ppneumonia, emphysema, cystic fibrosis, shallow breathing or holding your breath. – Metabolic Acidosis • Characteristics – low blood pH; normal or low (if lungs are compensating) blood PCO2; low blood HCO3- • Causes – Presence of ketone bodies (acetone, acetoacetic acid, beta hydroxybuyteric acid) due to starvation or poorly controlled diabetes. • See Table 7.7 p. 244 and Figure 7.12 p. 245 Reducing [H3O+] • Lungs remove excess acid through increase in respiration rate – As the blood becomes more acidic, the respiratory center of the brain signals for faster breathing. – With faster breathing, CO2 is exhaled at a faster rate thus reducing the partial pressure of carbon dioxide (PCO2). This reduces the [carbonic acid] thus reducing the [hydronium] producing an increase in pH. – This happens when you exercise. • Lungs remove excess base by reducing rate of respiration. – Breathing becomes slower and more shallow. PCO2 increase leads to increase [carbonic acid] and thus [hydronium] and a drop in pH. Reducing [H3O+] Cont’ • Kidneys remove excess acid by releasing bicarbonate into the blood. – The increase in [bicarbonate] shifts the equilibrium toward carbonic acid. This reduces the [hydronium]. Correcting Acidosis CO2 + H20 ↑ respiration rate (breathing becomes more rapid) causes ↓ pCO2 (lungs remove carbon dioxide from blood and release it into atmosphere) shifts equation. Think about exercise ↔ H2CO3 ↔ H3O+ + HCO3- kidneys release H3O+ in urine. kidneys generate / release bicarbonate shifts equation shifts equation Alkalosis • Alkalosis - high blood pH. • Leads to headaches, nervousness, cramps, and convulsions and death. – Respiratory alkalosis • Characteristics – high blood pH; low blood PCO2; normal or lower (if kidneys are compensating) blood HCO3- • Causes – Occurs when CO2 is exhaled from the body more quickly than it is produced by cells. – Hyperventilation brought on by anxiety, CNS damage, aspirin poisoning, fever, etc – Metabolic alkalosis • Characteristics – high blood pH; normal or high (if lungs are compensating) blood PCO2; high blood HCO3- • Causes – Excessive use of antacids and constipation. • See Table 7.7 p. 244 and Figure 7.12 p 245 Correcting Alkalosis CO2 + H20 ↔ H2CO3 ↓ respiration rate (breathing slows) causes ↑ pCO2 shifts equation. ↔ H3O+ + kidneys generate and release acid into blood shifts equation HCO3kidneys remove HCO3from blood and release it into urine. shifts equation