chemical formulas
advertisement

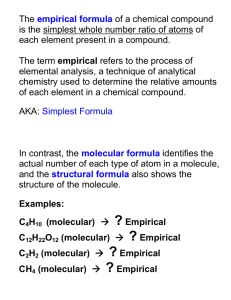
Empirical Formula – a smallest whole number ratio Ionic compounds only use an Empirical Formula Sugar Salt Molecular Formula – tells exactly which and how many atoms are attached to each other in a molecule of something Molecular compounds can use a Molecular Formula and an Empirical Formula A chemical formula is a ratio of moles: KBr means that for every mole of potassium bromide, there is 1 mole of K and 1 mole of Br H2O means that for every mole of water, there are 2 moles of H and 1 mole of O If table salt was chemically analyzed and it was determined that a 58.44 g sample of salt contains 22.99 g of Na and 35.45 g of Cl, what is the empirical formula of this compound? How many moles of each element were in the sample? What is the ratio of Na moles to Cl moles in the sample? What is the empirical formula for this sample? A mass spectrometer is a device that can blast compounds apart into their pieces and collect mass data for the sample. Mass data can be used to determine empirical and molecular formulas for unknown compounds. 1. Turn mass data into moles of each element 2. Find the smallest whole number ratio A mass spectrometer is used to analyze an ionic compound containing only lithium and fluorine. A 38.9 g sample is determined to have 28.49 g of fluorine and the rest lithium. What is the empirical formula of this compound? Why must you find a WHOLE NUMBER ratio? Percent Use 100g Mass Use molar mass Moles Divide all by smallest number; multiply all by whole numbers as needed Molecular Formula Use molar mass of compound Empirical Formula Smallest whole number ratio (Percent)MassMolesSmallest-whole-number-ratio (divide all by smallest number, multiply all by whole numbers as needed)Empirical formula(Molecular formula) A compound containing only sulfur and oxygen is decomposed and analyzed for mass data. The entire compound had a mass of 1.440954 g and 0.48099 g of sulfur was isolated from the compound. Find the empirical formula for this compound. (Percent)MassMolesSmallest-whole-number-ratio (divide all by smallest number, multiply all by whole numbers as needed)Empirical formula(Molecular formula) A compound is decomposed and analyzed for mass data. The compound is composed of 7.2066 g C and 1.51185 g H. In a separate experiment, the compound is found to have a molar mass of 87.2 g/mol. Find the empirical and molecular formulas for this compound. (Percent)MassMolesSmallest-whole-number-ratio (divide all by smallest number, multiply all by whole numbers as needed)Empirical formula(Molecular formula) A compound is analyzed for composition and found to be 23.76% S, 52.53% Cl, and the rest oxygen. What is the empirical formula for this compound? A compound is analyzed for composition and found to be 23.76% S, 52.53% Cl, and the rest oxygen. What is the empirical formula for this compound? (Percent)MassMolesSmallest-whole-number-ratio (divide all by smallest number, multiply all by whole numbers as needed)Empirical formula(Molecular formula) A hydrated compound, FeSO4.xH2O, was heated carefully to remove the water without decomposing the compound. The initial compound had a mass of 16.46 g. After the water was removed the compound had a mass of 12.14 g. Find the empirical formula for the hydrated compound.