Stoichiometry - Warren County Schools
advertisement

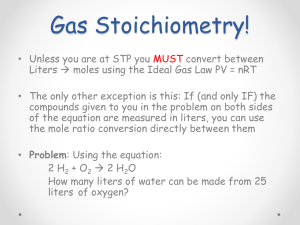
Stoichiometry Objectives: • Identify what stoichiometry is in chemistry. • Apply stoichiometry calculations in chemical reactions. • Identify and apply limiting reagents in chemical reactions. • Calculate % yield of products in a chemical reaction. Chemistry: 5.5 Due: Percent Composition Worksheet (late) Objectives: Complete Chemical Quantities Exam Define stoichiometry Homework: Define stoichiometry. Chemistry: 5.6 Infinite Campus Update: • Percent Composition Worksheet(10pts.) • Bubble Gum Lab (10pts.) Objectives: • Identify and apply stoichiometry with substances in a chemical equation. (mole to mole) Homework: Stoichiometry problems: mole to mole conversions What is Stoichiometry? access.aasd.k12.wi.us Stoichiometry N2 + 3H2 ----------> What is stoichiometry?: (NH3) www.larapedia.com 2NH3 (N2) N2 Stoichiometry + 3H2 ----------> (NH3) www.larapedia.com 2NH3 (N2) Chemistry: 5.8.14 Due: • Stoichiometry Problems (moles to mass) Objectives: • Identify and apply stoichiometry with substances in a chemical equation. (mole to mole and mass to mass) • Stoichiometry Gallery Walk Homework: Quiz tomorrow over stoichiometry conversions. Stoichiometry Lab Stoichiometry Gallery Walk Chemistry: 5.9.14 Due: • Stoichiometry Problems (mass to mass) Objectives: • Identify and apply stoichiometry with substances in a chemical equation. (mole to mole and mass to mass) • Stoichiometry Quiz Homework: Stoichiometry Problems Stoichiometry www.larapedia.com Stoichiometry N2 + 3H2 ----------> 2NH3 Stoichiometry: • Converting between substances in a balanced chemical equation using mole conversions. (NH3) www.larapedia.com (N2) Chemistry: 5.7 Posted in Infinite Campus and LATE if not turned in! • Percent Composition Worksheet(10pts.) • Bubble Gum Lab (10pts.) Objectives: • Identify and apply stoichiometry with substances in a chemical equation. (mole to mole and mass to mass) Homework: Stoichiometry problems: mass to mass Stoichiometry: Bell Ringer H2O ----------> H2 + O2 1. What type of reaction is this? 2. Balance the chemical equation if needed. 3. If you start with 100 moles of H2O how many moles of H2 would be produced? 3. If you want to produce 3.8moles of O2, how many grams of H2O would you need to start with? Stoichiometry www.larapedia.com Stoichiometry www.larapedia.com Stoichiometry www.larapedia.com Stoichiometry Problems N2H4 + • O2 -----> N2 + 2 H2O If 13.8 grams of water were produced how much rocket fuel (N2H4) in grams would have been used up? Chemistry (4/29) Objectives: • Complete post-lab questions for Hydrate Lab. • Identify and apply stoichiometry with substances in a chemical equation. Homework: Stoichiometry Problems Cu(SO4) . Hydrate Lab H2O ----> Cu(SO4) + H2O Post-Lab questions: Use the equation above to help answer the following questions: 1. What was the hydrate salt in this experiment? 2. What was the anhydrous salt in this experiment? 3. What was the difference between the hydrated salt and the anhydrous salt in this experiment? 4. Was this a physical or chemical reaction? Validate your answer with data from your lab. Cu(SO4) . Hydrate Lab H2O ----> Cu(SO4) + H2O Copper Sulfate Hydrate Chemistry (5/1) Infinite Campus Update: • Chemical Quantities Exam (37pts) • Hydrate Lab (10pts.) Objectives: • Identify and apply stoichiometry with substances in a balanced chemical equation. Homework: Stoichiometry Problems Bell Ringer 1. Calculate the percent composition of water in this hydrate: Mg(SO4) . 7H O 2 (Epsom Salt) 2. What is stoichiometry? 3. N2H4 + O2 -----> N2 + H2O a. Balance the chemical equation. b. How many moles of rocket fuel (N2H4) are needed to produce 8 moles of N2? Bell Ringer 1. Calculate the percent composition of water in this hydrate: Mg(SO4) . 7H O 2 (Epsom Salt) 2.What is stoichiometry? 3. N2H4 + O2 -----> N2 + H2O a. Balance the chemical equation. b. How many moles of rocket fuel (N2H4) are needed to produce 8 moles of N2? Bell Ringer 1. Calculate the percent composition of water in this hydrate: Mg(SO4) . 7H O 2 (Epsom Salt) 2. What is stoichiometry? 3. N2H4 + O2 -----> N2 + H2O a. Balance the chemical equation. b. How many moles of rocket fuel (N2H4) are needed to produce 8 moles of N2? Stoichiometry N2 + 3H2 ----------> 2NH3 Stoichiometry: • Converting between substances in a balanced chemical equation using mole conversions. (NH3) www.larapedia.com (N2) Stoichiometry H2O ----------> H2 + O2 1. What type of reaction is this? 2. Balance the chemical equation if needed. 3. If you start with 100 moles of H2O how many moles of H2 would be produced? 3. If you want to produce 3.8moles of O2, how much H2O will you need to start with? Chemistry (5/2) Objectives: • Identify and apply stoichiometry with substances in a balanced chemical equation. Homework: Stoichiometry Problems Chemistry (5/6) Objectives: • Identify and apply stoichiometry with substances in a balanced chemical equation. Homework: Stoichiometry Problems (due Wed.) Gas Laws Graphing Assignment (due Wed.) Quiz: Wednesday (Stoichiometry conversions: mole to mole or grams to grams.) Stoichiometry N2 + 3H2 ----------> 2NH3 Stoichiometry: • Converting between substances in a balanced chemical equation using mole conversions. www.larapedia.com Stoichiometery Problems Stoichiometry Lab Na(HCO3) + H(C2H3O2) --> CO2 + H2O + Na(C2H3O2) Objectives: • Complete lab and answer post lab questions. • Pass-out/ review study guide questions Homework: • Review for exam-test Wed. Stoichiometry Lab Data Group # 1 2 3 4 5 6 7 8 Theoretical Actual Yield Yield Na(C2H3O2) Na(C2H3O2) % Yield of Na(C2H3O2) Stoichiometry: Limiting Reactants • Limiting Reactant: • Excess Reactant: en.wikipedia.org Stoichiometry: Limiting Reactants • Limiting Reactant: completely consumed • Excess Reactant: partially consumed en.wikipedia.org Stoichiometry: Limiting Reactant HCl + Mg -------> MgCl2 + H2 1. What type of reactions is this? 2. Balance equation if needed. 3. If 6.8 moles of Mg react with 7.5 moles of HCl which is considered the limiting reactant? excess reactant? Reactants Have (moles) Need (moles) Stoichiometry: Limiting Reactant 2HCl + Mg -------> MgCl2 + H2 1.If 6.8 moles of Mg react with 7.5 moles of HCl how many moles of MgCl2 can be produced? Reactants Have (moles) Need (moles) HCl (limited) 7.5 mol 13.6 Mg (excess) 6.8 mol 3.75 Theoretical vs. Actual Yield of Products • Theoretical yield: maximum amount that can be produced according to the limiting reactant. (calculated yield) • Actual yield: actual amount produced in the lab. Stoichiometry: Limiting Reactant 4. Limiting Reactant: Al Theoretical Yield of AlCl3: 3.0 moles 5. Limiting Reactant: C2H4 Theoretical yield of H2O: 5.4 moles 6. Limiting Reactant: N2 Theoretical yield of NH3: 34 g Limiting Reactant Stoichiometry: Percent Yield 4. Theoretical Yield of AlCl3: 3.0 moles Actual yield from lab of AlCl3: 2.8 mole % Yield of AlCl3 : 5. Theoretical yield of H2O: 5.4 moles Actual yield from lab of H2O: 4.9 moles % Yield of H2O: 6. Theoretical yield of NH3: 34 g Actual yield from lab of NH3: 31.5 g % yield of NH3: Stoichiometry: Percent Yield % Yield of Product: Accuracy of product formation in the lab. % Yield of product: actual yield x 100 theoretical yield Actual yield: actual amount formed in lab Theoretical yield: maximum amount that can be produced according to the limiting reactant. (calculated yield) Pre-AP Chemistry 4/26 Objectives: • Identify and apply limiting reactants in stoichiometric calculations. • Distinguish between theoretical and actual yield. • Calculate % yield. Homework: Limiting and % Yield worksheets. Pre-AP Chemistry (4/29) Objectives: • Identify and apply limiting reactants in a chemical equation. • Calculate percent yield of products in a chemical equation. • Identify what molarity is. Homework: • Complete Limiting and % Yield worksheet. • Read over lab and complete hypothesis section. *Quiz: Wednesday over stoichiometry, limiting reactants, and percent yield of products. Bell Ringer: Limiting Reactants and % Yield 1. 2. a. b. c. C2H2 + O2 --- CO2 + H2O Balance the equation if needed. If 2.40 moles of C2H2 reacts with 7.40 moles of O2 how many grams of water can be produced? What is the limiting reactant? Use limiting reactant to calculate theoretical yield of H2O in grams. If the actual yield of H2O in the lab was 40.1 grams, calculate what the % yield of H2O would be.