novinky v léčbě chronického srdečního selhání na prahu
advertisement

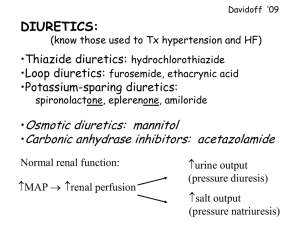
EDEMATOUS STATES AND RENAL DISEASE Jan Bultas Jitka Patočková Ústav farmakologie, 3. LF UK, Praha 2009 Edema – the main reasons • Cardiac failure (failure as a „pump“) - left ventricle (pulmonary edema) - right ventricle (edema of lower extremities, ascites, hydrothorax) • Failure of water excretion (from renal reasons, extrarenal – e.g. hormonal reasons etc.) • Decrease of oncotic pressure - hypalbuminemia (proteinuria, protheonsynthesis failure – hepatopathy, …) • Venous drainage failure (venous insufficiency, phlebotrombosis, etc.) • Lymphatic drainage failure HOW TO AFFECT WATER AND IONS RETENTION ? The best… ….According to the reason (Causal Therapy) ● Systemic edema ● Local edema (no diuretics!) DIURETICS Drugs affecting water and ions retention DIURETICS • Heterogenous group of drugs which are able to increase diuresis and excretion of electrolytes • Main indications: • fluid retention (heart failure, pulmonary congestion, edemas, ascites, hydrothorax) • hypertension • Main clinical effects: • in heart failure they improve the quality of life, but we do not have enough information about their influence on the life prognosis • In hypertension they decrease the incidence of cerebral and myocardial attacks, they decrease mortality DIURETICS – mechanism of action • Transport proteins inhibition (inhibition of transport of ions in tubular system) (loop diuretics, thiazides, potassium sparing diuretics) • Glomerular filtration increase (osmotic diuretics, methylxantines) • Aldosterone inhibition (aldost. receptors blocators) or vasopressin inhibition (vasopres. receptors blocators – „aquaretics“, ev. alcohol) DIURETICS – main groups Henle´s loop diuretics Distal tubule diuretics (thiazides) Potassium-sparing diuretics Mechanism of action of diuretics osmotic diuretics, methylxantines Triamterene, amiloride Na+ Na+, H2O Carbonic anhydrase inhibitors osmotic diuretics H2O Na+ Clthiazides, indapamid Na+ K+ Na+ Clloop diuretics Aldost. receptors antagonists H2O „aquaretics“ LOOP DIURETICS Na+/K+/2Cl- co-transport inhibition in Henle´s loop (- increase of ion (Na, K, Mg, H) and water excretion) • Furosemide: huge diuretic effect, quick onset, short biological half life (1,5 hr), suitable for patients with significant renal failure, variable bioavailability . In patients with chronic heart failure, wide dose range 20mg -2g, not suitable as antihypertensive drug (short half life) • torasemide: better profile, longer diuretic effect, stable bioavailability, enormous price • bumetanide, ethacrynic acid – not used LOOP DIURETICS ADVERSE EFFECTS • Potassium depletion, hypokalemia • hyponatremia, hypomagnesaemia, hypovolemia Glomerular filtration decrease in hypovolemia • ototoxicity • Increase of nephrotoxicity of a lot of nephrotoxic drugs (i.e. ATB) DISTAL TUBULE DIURETICS - THIAZIDES Na+/Cl- co-transport inhibition in distal tubule • Weaker diuretic effect, slow onset, long biological half life, stable bioavailability, narrow therapeutic window, • No effect in patients with glomerul. filtration decrease (no effect in patients with renal insufficiency) • Loop diuretics effect potentiation (convenient combination) • Basic antihypertensives • hydrochlorothiazide (6-12 hr, 6,25-25 mg), chlorthalidon (48-72 hr, 6,25-25 mg) • indapamid: also vasodilatation (16-36 hr, 2,5 mg) DISTAL TUBULE DIURETICS - THIAZIDES ADVERSE EFFECTS • Potassium depletion, hypokalemia • hyponatremia, hypovolemia, hypotension • metabolic effect in higher dosage: - glycid and lipid metabolism disturbances, hyperurikemia • Clear tendency to use doses, • Be careful in diabetics POTASSIUM - SPARING DIURETICS Direct antagonism of mineral corticoid receptors (spironolactone) Na+ flux inhibition in collecting tubule ( triamterene, amiloride) • amiloride: weak diuretic effect, slow onset, long biological half life (days), suitable for combinations (with loop diuretics, thiazides), as antihypertensive drugs and in the treatment of heart failure • triamterene: less profitable, shorter effect a combination of loop diuretics with potassium-sparing diuretics improve a life prognosis of the patients in comparison to the use of loop diuretics only AE : hyperkalemia Aldosterone receptors Aldosterone rec. in distal tubule mineral corticoid effect (Na+/K+ exchange) Aldosterone rec. in myocardial tissue Stimulation of fibro-proliferation Aldosterone rec. in smooth muscles of blood vessels and endothelium Stimulation of fibro-proliferation Aldosterone receptors antagonists Na+ Na+ ClNa+, H2O Na+ Aldosterone receptors K+ antagonists Na+ ClH2O H2O SPIRONOLACTONE aldosterone receptors antagonism in: • heart muscle: inhib. of fibroblast proliferation ( doses 25 mg/d) • kidneys: inhib. of Na/K pump in distal tubule – kalium retention and natriuresis ( doses 50-300 mg/d) • androgen-like effect (gynecomastia, menstruation disturbances) • Active metabolite with longer half life (15 hr) • Cave! Risk of hyperkalemia EPLERENONE • Myocardial and renal effect - same as spironolactone • No androgen-like effect, better tolerated, expensive Aldosterone receptors antagonists indication • Chronic heart failure (decrease of mortality to 75%), „sub diuretic“ doses, main effect is prevention of hyperplasia of fibrous tissue in heart and vessels, combination with ACE-I, beta blockers, cardiac glycosides and diuretics • hyperaldosteronismus (higher dosage) • cilium depletion and its prevention (medium dosage) Diuretics indication Loop diuretics • Acute and chronic cardiac failure • Massive fluid retention, ev. retention in renal failure Thiazides • Antihypertensives of first choice • In combination with loop diuretics in poor diuretic response Potassium-sparing diuretics • In combination with saluretics (loop d., thiazides) • Kalium depletion AFFECTING RENIN-ANGIOTENSINALDOSTERON SYSTEM RAA System • Main role: keeping the body fluid volume and BP • AT1 receptors: vasoconstriction thirst stimulation retention of fluids and Na in kidneys natriuretic pept. release stimulation aldosterone stimulation • AT2 receptors: vasodilation • AT3 receptors : trombocytes activation • RAA system is in balance with the kinin system and natriuretic peptide RAA system activation - short time and long time effects ACUTE ADAPTATION CHRONIC MALADAPTATION increase of the volume of natrium and water retention fluid in circulation perif. vasoconstriction (to keep heart output and perfusion pressure) maintenance of circulation myocytes necrosis and apoptosis fibrous tissue proliferation fibrinolysis inhib. PAI-1 sympathic syst. activation break down of circulation Renin-Angiotensin-Aldosterone System angiotensinogen RENIN angiotensin I ACE angiotensin II rec. AT1 aldosteron Fibroblasts proliferation ANP,BNP thirst Na+ resorp. vasoconstriction Na+ retention Renin-Angiotensin-Aldosterone System angiotensinogen RENIN angiotensin I ACE angiotensin II AT inhibitores Beta- blockers, Renin inhibitores ACE Inhib. rec. AT1 aldosteron. rec. antagonists aldosterone Fibroblasts proliferation ANP,BNP thirst Na+ resorp. vasoconstriction Na+ retention ACE-INHIBITORS – MECHANISM OF ACTION: 1) Inhibition of A I to A II conversion 2) Inhibition (slow down) of bradykinin degradation Pharmacodynamic: - decrease of peripheral vessels resistance - specific dilation of vas efferens (intraglomerul. pressure decrease, GF decrease) - decrease of ALDOSTERONE and ADH release + thirst suppress (decrease of Na and H20 retention) - decrease of NORADRENALIN release - Stimulation of fibrinolysis - Antimitogenic activity + inhibition of apoptosis “ Chalk and talk“ NSAD´s effect on vas afferens (constriction) Indication of ACE-I • arterial hypertension • Chronic heart failure • Prophylaxis of nephropathy progression (specially Diabetic nephropathy ) • For better prognosis and decrease of morbidity in patients with cardial ischemia and after cerebral attack Adverse effects and contraindications of ACE-I AE: • cough (cca in 5% of patients should discontinue a drug) • angioedema (less than 1%) • hypotension, first dose phenomenon (kaptopril, hypovolemia) • Worsening of renal function (decrease of intraglom. pressure) • hyperkalemia • KI: • gravidity!!! • bilat. stenosis of ren. Art., signif. AO stenosis and obstructive cadiomyopathy Widely used ACEI Enalapril Fosinopril Imidapril Lisinopril 2x 5-20 mg 1x 10-20 mg 1x 5-10 mg 1x 20-80 mg Moexipril Perindopril Quinapril 1x 7,5-15 mg 1x 4-8 mg 1-2x 5-20 mg Ramipril Spirapril Trandolapril 1x 2,5-10 mg 1x 6 mg 1x 2-4 mg Influence on mortality in patients after myocardial infarction – different ACEI (ONTARIO) 100% 90% ramipril perindopril lisinopril enalapril quinapril fosinopril kaptopril 80% 0 6 12 měs. Pilote L et al, Ann Intern Med, 2004 AT1 receptor antagonists, angiotensin II receptor antagonists ARB´s „sartans“ Renin-Angiotensin-Aldosterone System angiotensinogen RENIN angiotensin I ACE angiotensin II rec. AT1 aldosterone Fibroblasts proliferation ANP,BNP thirst Na+ resorp. vasoconstriction Na+ retention Differences in Effect of AT1 blockers and ACE-I BK AII ACE ACE-I ACE natriuréza vazodilatace stimul. NOS ACE-I Differences in Effect of AT1 blockers and ACE-I ACE AII ARB rec. AT1 ANP,BNP thirst resorp. Na+ vasoconstriction retence Na proliferation rec. AT2 rec. AT3 aktivace trombocytů antiproliferativní vazodilatace stimul. NOS Effects of AT1 receptor antagonists • vasodilation, decrease of peripheral resistency (weaker effect than in ACE-I, there is no effect on bradykinin) • decrease of fluid retention • inhibition of LV remodelation ( apoptosis, necrosis) • suppression of sympaticotonia • specif. dilation of vas efferens (decrase of intraglom. pressure) • there is no evidence of the effect on correction of edothelial dysfunction Advantages and disadvantages of AT1 rec. antagonists advantages disadvantages • better tolerability (less • weaker effect on vasodilation and antihypertensive effect (no effect on bradykinin) cough and angioedema) Indication of AT1 receptor antagonists • Arterial hypertension • Profylaxis of nephropathy progression (spec. diabetic) • Better prognosis and decrease of mortality in patients with cardiac ischemia and in patients after stroke • Chronic cardiac failure AE and contraindications of sartans AE: • hypotension, (namely in hypovolemia) • Worsening of renal function (decerase of glom. pressure) • hyperkalemia • Cough and angioedema (rarely) CI: • gravidity!!! • bilat. Stenosis of renal art., significant AO stenosis, obstructive cardiomyopathy Therapy of venous insufficiency • Pathogenesis - increase of venous pressure in distal part of venous system (LE namely) • Participation of inflammatory agents • Therapy is focused on profylaxis and regime changes • Pharmacotherapy has limited importance, there is some effect on decrease of pain, cramps, heaviness, and in some cases acceleration of varicose ulcers Pharmacotherapy of venous insufficiency • Natural substances (flavonoides) – diosmine, hesperidine, aescin etc. • semisynthetic – troxerutin • synthetic – calcii dobesilat etc. • The combination diosmine + hesperidine seems to be the most evidential (EBM) PHARMACOTHERAPY IN RENAL DISEASE Renal excretion of drugs and their metabolites a) Glomerular filtration b) Tubular secretion c) Passive Tubular diffusion Glomerular filtration • Filtration of molecules (drugs) up to molecular weight 20 000 • Hydrophilic drugs - mainly free filtration • Lipophilic drugs - binded to albumin, just free fraction is filtrated (cca 2%) • cca 20% of renal perfusion – i.e.. Cca 20% of drug excretion Tubular secretion and passive diffusion • 80% of renal perfusion - plasma / drug – in peritubular capillary syst. • In prox. Tubulus the transport systems excrete xenobiotics to urine • Tubular secretion is the most effective system in drug clearance • Lipophilic molecules move freely through the tubular wall and back to plasma (diffusion) • Hydrophilic molecules (ionized) – no diffusion • pH changes influence significantly drug ionization – drug excretion Drug dosage in renal failure • Drug dosage (in acute and chronic treatment) – Loading (first) dose – without reduction – Maintenance (repeated) dose - reduced • Dosage reduction information – SPC, AISLP, Cocroft Gault formula, etc. • TDM in drugs with narrow therapeutic window and renal excretion only – Digoxin, amino glycosides, vancomycin Drug dosage in renal failure - examples Antihypertensive drugs: – No reduction: calcium channel blockers, AT1 antagonists, Lipophilic beta blockers (metoprolol), furosemide – Mild reduction: ACE-I (to half of normal dosage) – Reduction according to GF: hydrophilic BB (atenolol, bisoprolol) • • • • • • statines: no reduction insulin: cave: biol. half time prolongation, higher effect! Opioid analgesics: biol. Half time prolongation (tramadol) NSAIDs: different / according to molecular structure corticoids: no reduction digoxin: reduction according to GF, in renal failure up to 0,125 mg 2x weekly – Always consider the indication and do TDM! Dosage of ATB in renal failure • Penicillin ATB + betalactamase inhibitors (Augmentin, Unasyn) – Prolongation of dosage interval to double • cephalosporines, chinolones – Prolongation of dosage interval to double • macrolides – no reduction • Co-trimoxazol - Prolongation of dosage interval to double • aminoglykosides – Significant reduction (see guidelines), always TDM • vancomycin – Significant reduction (see guidelines) – Dosage interval up to 7 days – Always TDM Diuretics in Treatment of renal failure • • • • Decrease of GF is not an indication of diuretic treatment thiazides – thiazides only - no effect in GF < 0,5 ml/s - in combination with loop diuretics – in any GF Loop diuretics – ekvipotent dose increases exp. with the GF reduction Diuretic treatment indication: – Fluid retention / edema – Heart failure with fluid retention – hyperkalemia – Antihypertensive therapy Severe AE of drugs in renal failure hyperkalemia ACE-I, AT1 rec. antagonists, spironolaktone, amiloride, KCl decrease of GF ACE-I, AT1 rec. antagonists, NSAID Cave: in renal perfusion disturbances take care about blood pressure (be careful in hypotension) Drug nephrotoxicity in renal failure • Higher risk in • Renal hypoperfusion • Risk combinations (spec. with NSAID) • Elderly people - Possibly irreversible damage! • Potentially nephrotoxic drug – – – – Consider indication! discontinue other potentially dangerous drugs After good hydration Renal function control afterwards Drug nephrotoxicity- examples – Rtg contrast substances – aminoglykosides – Some other ATB (cotrimoxazol, cephalosporines, vancomycin) – NSAIDs (significant ren. Vasoconstriction in prostanoid synthesis inhibition) – ACE-I and AT1 antagonists – GF decrease, mild worsening of renal function, but nevertheless nephroprotective effect Drugs contraindicated in renal failure • Oral antidiabetics – metformine – absolutely contraindicated – Most of sulphonylurea derivates • nitrofurantoin • Aldosterone receptor inhibitors (spironolaktone, eplerenone) Mistakes in pharmacotherapy in renal failure • Renal failure is not recognized • Potentially risk combination is given without any control • AE of NSAID are not enough considered • Dehydration is not enough considered • Treatment with loop diuretics where is no indication for them THANK YOU FOR YOUR ATTENTION IMMMUNOSUPRESSANTS Immunosupression The Aim: • To diminish selectively immune activity, ie. selective depression of T and B lymphocytes activation • To keep nonspecific immunity, ie. To keep the function of Polymorphonuclears and monocytes/macrophages Indications: • To reach transplant acceptation and to keep resistance against infection and tumor growing • To decrease activity of diseases based on immunoalteration Immunosupression Mainly combination of • Substances with complex antiinflammatory effect cortiocoids • Substances with antimetabolit effect – mycophenolat, azathioprim • Substances inhibiting transfer of activating signal in T lymphocytes – cyclosporin A, tacrolimus, rapamycin • Lymphocyte antibodies (antilymphocytes) Glucocorticoids • Anti-inflammatory effect: depression of function of Tlymphocytes and mono/macrophages, depression of (inflammatory) cytokines production, decrease of vascular permeability, depression of fibroprolipheration,… • Metabolic effect: - Decrease of glucose utilization - Increase of gluconeogenesis - hyperglycemia - Increase of protein degradation (catabolism) - Redistribution of fat • Bio-feedback: decrease of glucocorticoids production Cyclosporin A • Inhibition of signal transfer from activating receptor on T-lymphocyte to nucleus – block of cytokines synthesis and secondarily also Tlymphocytes activation • Cyclic peptide, very potent immunosuppressant • Significant nephrotoxicity, vasoconstriction Tacrolimus, rapamycin • Similar mechanism of action in T- lymphocytes • Very potent immunosupression • Better tolerability, rapamycin is not nephrotoxic