Acute Respiratory Distress Syndrome
advertisement

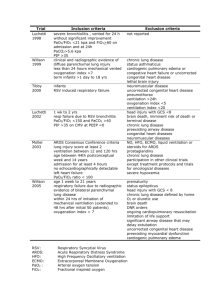
Acute Respiratory Distress Syndrome: The Berlin Criteria An Update On Clinical Guidelines DAVID AYMOND, MD ARDS History The first definition of ARDS dates to Ashbaugh and colleagues in 1967 when they described 12 patients with severe acute respiratory failure. They noticed the pts had severe hypoxemia that was refractory to supplemental oxygen, but which in some cases was responsive to the application of PEEP. At autopsy they observed widespread pulmonary inflammation, edema, and hyaline membranes. In 1994 the American-European Consensus Conference (AECC) defined ARDS. They redifined in 2000. The ARDS Conceptual Model: Path0physiology Defined by Clinicians Face validity derives from an understanding of how we recognize the syndrome, therefore, considerable discussion at the Berlin consensus focused on developing a conceptual model of ARDS The panel agreed that ARDS is a type of acute diffuse lung injury associated with a predisposing risk factor, characterized by inflammation leading to increased pulmonary vascular permeability and loss of aerated lung tissue. The hallmarks of the clinical syndrome are hypoxemia and bilateral radiographic opacities (using stnadard CXR or CT scan), associated with several physiological derangements including: increased pulmonary venous admixture (intra-pulmonary shunt), increased physiological dead space, and decreased respiratory system compliance [Vt/(PP-PEEP)] Morphological hallmarks in the acute phase are lung edema, inflammation, hyaline membranes, and alveolar hemmorhage (i.e. diffuse alveolar hemorrhage=DAD) AECC Definition American-Euro Consensus Conference (AECC) In an appropriate clinical setting (i.e. a likely underlying cause) There is an acute onset (< 72hrs) of bilateral alveolar and/or interstitial infiltrates on frontal chest radiograph A ratio of the Pa02 divided by the Fi02 < 300 (ALI) or < 200 (ARDS) Non-cardiogenic pulmonary edema = PCWP < 18 or no clinical evidence of increased LAP Why Was An Update Needed? AECC Pitfalls: Hypoxia defined as PaO2/FiO2 ratio. This is a problem because the FiO2 varies depending on the PEEP (Gowda 1997, Ferguson 2004) Wedge Pressure: Patients with ARDS may have elevated PAWP b/c of transmitted airway pressure and /or vigourous fluid resuscitation (ARDSnet 2006) CXR criterion has only moderate inter-observer reliability even when applied by experts Definition has a sensitivity of 84% but a specificity of only 51%. This was shown by autopsy of patients with lung biopsy. ALI is under-recognized by clinicians, especially those with very minor ALI The AECC definition says the onset of respiratory failure should be acute, but doesn’t define a time ARDS-Defined Berlin Criteria Timing: Within 1 week of a known clinical insult or new or worsening respiratory symptoms Chest Imaging: Bilateral opacities on x-ray or CT scan not fully explained by effusions, lobar/lung collapse, or nodules Origin of Edema: Respiratory failure not fully explained by cardiac failure or fluid overload. Need objective assessment (Echo and CVP) to exclude hydrostatic edema if no risk factor for ARDS present Oxygenation: Mild: Pa02/Fi02 of 201-300 mmHg with PEEP/CPAP >5 cm H2O (27%) Moderate: PaO2/FiO2 of 101-200 with PEEP > 5 cm H2O (32%) Severe: PaO2/FiO2 < 100 with PEEP > 5 cm H2O (45%) ARDS-Defined (cont) Berlin Criteria: 4 ancillary variables were included to help define severe ARDS 1. 2. 3. 4. Radiographic severity Respiratory System Static Compliance (Vt/Pplat-PEEP) PEEP >10 Corrected Expired Volume per Minute > 10L/min Results From Comparison of AECC and ARDS Definitions (JAMA.2012;307 (23): 5669) Meta-analysis of 4188 patients with ARDS from 4 multicenter clinical data sets and 269 patients with ARDS form 3 singlecenter data sets. This meta-analysis found, when compared with the AECC definition, the final Berlin Definition predicted duration of mechanical ventilation based on degree of hypoxemia, percent of shunt, and had better predictive validity for mortality. *The 4 variables were excluded from the definition of Severe ARDS after the meta-analysis found that they did not improve the predictive value for mortality Authors did a post-hoc “high risk profile” of patients with a 52% mortality from ARDS. These patients had severe ARDS and either a static compliance of < 20 cm H2O or a corrected expired volume of > 13 L/min What is the Origin of Edema Mean in the New Berlin Definition? The panel recognized that hydrostatic edema (Cardiogenic) is one of the most common alternative diagnoses in patients presenting with ARDS. However, given the declining use of Pulmonary Artery Catheters worldwide and the recognition that hydrostatic edema and ARDS may coexist, the pulmonary artery wedge pressure criterion was removed. The panel therefore decided that patients whose respiratory failure is not fully explained by cardiac failure or fluid overload as judged by the treating physician using all available data may qualify as having ARDS. Nevertheless, if no known etiologic RF for ARDS is apparent, objective evaluation of cardiac function is required to help R/O hydrostatic edema secondary to CHF. What did they define as established risk factors for ARDS? Known Etiologic Risk Factors Direct Causes Pneumonia (~40%) Aspiration (~15%) Near Drowning Inhalation Injury Lung Contusion Indirect Causes Sepsis (~25%) Traumatic Shock Non Cardiogenic Shock DIC Pancreatitis TRALI Drug OD Severe Burns Pulmonary Vasculitis Treatment Ventilation Primary goal is to prevent damage to undamaged alveoli The 4 potential mechanisms of alveolar damage in pts with ARDS: 1. Barotrauma: pressure 2. Volutrauma: over-distention of alveoli from high Vt ventilation 3. Atelectrauma: shearing force on alveoli from opening during inspiration and collapse on expiration 4. Biotrauma: pro-inflammatory cytokines releases from excessive mechanical forces on the lung In 2000, the ARDS network was established and they did a study that showed improvement in mortality (9%) with lung protective ventilation, spp. Lower Vt of < 6cc/kg of IBW and plateau pressure limits of 30 cm H2O Therefore current guidelines say to maintain low tidal volume ventilation < 6 mL/kg of IBW and to maintain Plateau Pressures < 30 cm H20; what variables affect plateau pressure? What is a plateau pressure? Ventilator Treatment (Cont) Current Guidelines are as follows (ARDS network) Vt < 6 mL/kg of IBW (keep pH > 7.15) Pplat < 30 cm H2O PEEP: titrate to a P plat of 28-30 cm H2O dec time on vent, better lung mechanics, and dec in mortality; if able to increase PEEP w/o inc Pplat suggests alveolar recruitability FiO2: increase PEEP rather than FiO2 (keep <0.6) I:E ratio goal: Duration of inspiration be < duration of expiration Set initial rate to approximate baseline minute ventilation (not more than 35 bpm) Adjust Vt and RR to achieve pressure goals and pH Treatment Details-Tidal Volume and pH Permissive Hypercapnia This is a term that was coined when low Vt started being used b/c the low Vt leads to respiratory acidosis from accumulating CO2 just consider the definition of minute ventilation= RR x Vt this is indirectly related to CO2, meaning if you increase either variable, CO2 goes down and vice versa. Current guidelines therefore suggest keeping pH > 7.15 and PaCO2 in the 45-55 mm HG range ARDS network set forth the following guidelines: pH Goal: 7.30-7.45 Acidosis management: pH < 7.3 f pH 7.15-7.30: increase RR until pH > 7.30 or PaCO2 < 25 (max set RR 35) If pH < 7.15: Increase RR to 35 • If pH remains < 7.15, Vt may be increased in 1 ml/kg steps until pH > 7.15 (Pplat target of 30 may be exceeded); consider giving NaHCO3 Alkalosis management: ph > 7.45: decrease vent rate if possible Treatment Details- Pplat Plateau pressure is the pressure that is applied to small airways and the alveoli Factors that contribute to the Pplat are Vt, PEEP, static compliance, and PS Is measured at the end of inspiration (inspiratory hold) Different from the peak pressures; peak pressures are the maximal pressures in the proximal airways at the end of each lung inflation. Pressure Support: at the onset of each spp breath, the negative pressure generated by the patient opens a valve that delivers the inspired gas at a pre-selected pressure (usually 5-10 cm H2O). The majority of this pressure is distributed at the proximal airway (trachea, main-stem bronchus) Treatment Details: PEEP PEEP PEEP helps recruit atelectatic lung tissue and prevents the development of further atelectasis; it thereby improves ventilation to perfusion matching. This is the same thing as decreasing the intra-pulmonary shunt Current guidelines suggest using PEEP to maintain oxygenation instead of FiO2, suggest to never go above 0.6 on FiO2, with the goal of getting FiO2 below 0.5 ASAP Supportive Therapy Paralysis Not currently recommended, although a study showed that if cisatracurium was started early in ARDS and only used for 48 hrs there was a decrease in mortality. This was due to the ability to provide better lung protective ventilation Nutrition Tube feeds with increased fat and decreased carbs should lead to less CO2 production and result in a decreased amount of respiratory acidosis. *Fluid management discussed on last slide ARDS Treatment (cont) Steroids: 1 - 7 days but ideally < 72 hrs: Methylprednisolone 1mg/kg IV bolus then 1mg/kg/day continuous IV infusion for 14 days; After 14 days decrease to 0.5mg/kg/day x 7 days, then 0.25mg/kg/day IV x 7 days, then stop If no clear physiologic or radiologic improvement in 3-5 days, d/c 7 - 14 days, if steroids not started earlier, benefit less certain, still try above protocol and if no benefit in 3-5 days d/c > 14 days, not recommended and may cause inc mortality Rescue Therapy Therapies that are not well proven but used for patients in extreme situations not responding to aforementioned therapy Alternative Vent modes: Positioning APRV: Airway pressure release ventilation maintains a constant airway pressure, recruiting more alveoli; no survival advantage HFO : ? Useful for pts who have severe hypoxemia and failed conventional vent ECMO: studies ongoing (currently no proven benefit in adults) Oxygenation improves in 2/3 of pts when placed in prone position by unloading weight of the heart and improved aeration of dorsal lung regions Recruitment Maneuvers A typical recruitment maneuver is an attempt at re-expanding atelectatic lung tissue by applying a constant positive pressure of 30-40 cm H2O for ~ 60 seconds; ROUTINE USE NOT SUPPORTED Outcomes Survival rates for ALI/ARDS are controversial. Some evidence suggests improved survival while a recent estimate of survival in the control groups of studies in ALI suggest that survival rates are unchanged (Am J Respir Crit Care Med. 2009; 179:220) Most pts that survive have abnl on PFT including muscle weakness, les distance walked in the standardized 6 min walk test, and in one study 6% had O2 saturation less than 88% with exercise A most recent study has found that 5 yrs after surviving ARDS the survivors had normal to near-normal results on their PFT, but they had significant exercise limitation with a decreased physical quality of life. As expected younger pats had a greater rate of recovery than older ones, but neither group returned to normal predicted levels of physical function at 5 yrs (N Engl J Med. 2011; 364:1293) Fluid Management in ARDS Target CVP 4-6 cm H20 (if nonoliguric and normotensive) This increases vent and ICU free days, but no effect on mortality (ARDS network FACTT trial) Accomplished with fluid bolus and furosemide bolus/infusion (20mg or 3mg/hr) Non-Oliguric = UOP > 0.5 cc/kg/hr