Protein Folding Activity - Fort Thomas Independent Schools
advertisement

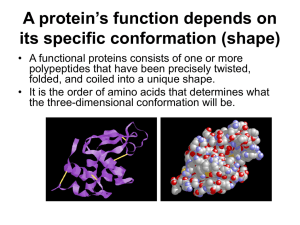
Understanding Proteins Primary Structure What did holding hands represent? What was your left hand representing? What was your right hand representing? Why did we rearrange the amino acids aka you? The holding of hands represents what? At the ends of our polypeptide chain, what did we have? Why did I pull you back and forth? What does this represent? Secondary Structure Why did we “bend” the line of amino acids? And why did your arms going up and down represent? What did the arms up reaching across the bend and connecting thumbs represent? Secondary Beta Pleated Sheet So arms up on one side were the double bonded C from the carboxyl end and the arms down were the hydrogen from the other sides amine group (the up and down are not relevant – just for the simulation). (I had to add the bend… use your imagination ) Secondary Structure Points to understand: The Hydrogen bonds form at regular intervals, between the backbone components. The Hydrogen bonds are “weak” compared to the peptide bonds. *The number of the H bonds in the entire structure does give strength to the structure (so alone H are weak, a lot of them are stronger) The Hydrogen bonds and the pleats create a regular structure from the amino acid chain. Note –can’t construct alpha helixes using students, but can you visualize this structure? Secondary Structure Alpha Helix Note this is not showing R groups Secondary Structure with both Alpha helix and Beta pleated sheet Tertiary Structure Points to understand: The “S” shaped hooks on the bungee cord represent sulfur atoms. The tertiary structure is formed when two sulfur atoms are joined bonded together by the strong non-polar covalent bond. S-S bonds form from the interactions between the side chains – “R” groups. The S-S bond is more rigid than Hydrogen bonds. This gives the protein structure more rigidity and stability. The size of the loops created by the S-S bonds depends on the location of the sulfur containing amino acids (primary structure). Tertiary Structure Quaternary Structure How did we represent this structure? The two or more small polypeptide chains. The chains could have been positioned side by side or in other configurations. A quaternary structure is formed when two or more polypeptides come together to make the functional protein. Points to understand: Two or more separate polypeptides are needed for this level of protein structure. Each polypeptide has its own primary, secondary and tertiary structure. Often these levels of structures provide the fit between the different polypeptides in forming the functional protein. Genetic conditions such as Thalasemia occur when the polypeptide units are not produced in equal amounts. The result is that one polypeptide chain lacks a sufficient number of partners to form the final functional protein unit. Quaternary Structure What determines a proteins shape? The sequence of amino acids determines the protein's shape - *** Why? The sequence determines where an a helix can form, where beta pleated sheets can occur, where disulfide bridges are located, where ionic bonds can form etc. What effects proteins? Genetics – example: forms of inherited autosomal recessive blood disorders that originated in the Mediterranean region. In thalassemia, the disease is caused by the weakening and destruction of red blood cells. Thalassemia is caused by variant or missing genes that affect how the body makes hemoglobin. Denature What does it mean to denature a protein? What factors cause denaturing? Misc. Is the environment inside a cell good for protein folding? In the crowded environment inside a cell, there are also specific proteins that aid in the folding of other proteins Chaperonins (or chaperone proteins) protein molecules that assist in the proper folding of other proteins (Figure 5.24). Chaperonins do not specify the final structure of a polypeptide. Instead, they keep the new polypeptide segregated from "bad influences" in the cytoplasmic environment while it folds spontaneously. The chaperonin shown in Figure 5.24, from the bacterium E. coli,is a giant multiprotein complex shaped like a hollow cylinder. The cavity provides a shelter for folding polypeptides. http://click4biology.info/c4b/7 /pro7.5.htm http://www.rothamsted.ac.uk/notebook/prot. htm#III Pictures and definitions from wikipedia and Campbell book Activity from J. Smith Indiana Academy http://video.about.com/chemistry/Introducti on-to-Amino-Acids.htm Above video shows why carboxyl is acidic and amine is basic* show just first part