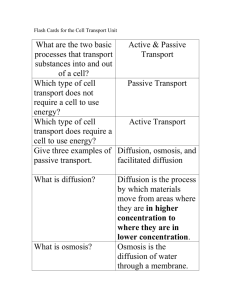
passive transport
advertisement
Passive Transport Unit 4, Lesson 2 Passive Transport Passive Transport is the movement of molecules across a membrane that does not require energy No energy is required because molecules are moving down a concentration gradient from areas of higher concentration to areas of lower concentration Types of passive transport: Diffusion Facilitated Diffusion Osmosis Diffusion The net movement of particles from an area of high concentration to an area of low concentration Diffusion results from the random movement of particles http://highered.mheducation.com/sites/9834092339/stud ent_view0/chapter38/how_diffusion_works.html Concentration Gradient The difference in the concentration of a substance over distance The bigger the difference between “high” and “low” areas of concentration, the steeper the gradient Results of Diffusion As substances continue to diffuse, they will eventually become evenly distributed. This is called dynamic equilibrium Molecules will continue to move across a membrane in both directions, since there is no “high” or “low” anymore Facilitated Diffusion Movement of molecules down a concentration gradient from areas of HIGH concentration to areas of LOW concentration with the help of transport proteins. Facilitated Diffusion Still passive transport because no energy is required (still moving from high concentration to low concentration) Larger molecules or charged molecules move via facilitated diffusion They can’t slip through the lipid bilayer directly, but they can go through these transport proteins Channel protein – like tunnels Carrier proteins – bind to molecules and carry them across http://highered.mheducation.com/sites/9834092339/student_v iew0/chapter38/how_facilitated_diffusion_works.html OSMOSIS Diffusion of water across a semipermeable membrane is called osmosis Water will move towards the areas where there are more solutes (and therefore less water) Water flow helps maintain homeostasis Osmosis Water moves TOWARDS the solutes Solutes = dissolved particles like salts and sugars Osmosis http://highered.mheducation.com/sites/9834092339/stud ent_view0/chapter38/how_osmosis_works.html Types of Solutions Isotonic = balanced concentration inside and outside the cell Water will move in and out equally Hypertonic =greater concentration of solutes outside the cell Water will move out of the cell (toward the solutes outside) Hypotonic = lower concentration of solutes outside the cell Water will move into the cell (toward the solutes inside) WATER MOVES TOWARDS THE SALTS/SUGARS Red Blood Cells Plant cells Water moves towards the solutes Types of Solutions http://highered.mheducation.com/sites/9834092339/stud ent_view0/chapter38/animation_-_osmosis.html