Balancing PPT
advertisement

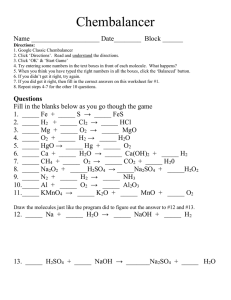
You Will Need: 1.Pencil, Text Book Periodic Table 3. Colored Pencils 4. Balancing Equations #1(2 sheets/4 sides) (Pick-up from bookshelf- Please Staple 5. Counting Total Atoms in Compounds – Due today Vocabulary Word Catalysts Inhibitors Definition Starts and/or Speeds Up Chemical Reactions Stops and/or Slows Down Chemical Reactions Word Conservation of Mass Reactants Products Coefficient Definition Same amount of particles before and after every reaction. 2 NaOH + H2SO4 Na2SO4 + 2 H2O Conservation of Mass = the same number of atoms of each element on each side of the equation. Word Definition Conservation of Mass Same amount of particles before and after every reaction. Reactants Substances going into the reaction. Products Coefficient 2 NaOH + H2SO4 Na2SO4 + 2 H2O Reactants = the substances going into a reaction Word Definition Conservation of Mass Mass can neither be created or destroyed in any chemical reaction. Reactants Substances going into the reaction. Substances produced by the reaction Products Coefficient 2 NaOH + H2SO4 Na2SO4 + 2 H2O Products = the substances being produced by the reaction Word Conservation of Mass Reactants Definition Mass can neither be created or destroyed in any chemical reaction. Substances going into the reaction. Products Substances produced by the reaction Coefficient The number in front of the formula indicating how many molecules 2 NaOH + H2SO4 Na2SO4 + 2 H2O Coefficient = The number in front of each formula or element indicating how many molecules 1. Get Yourself an unbalanced equation. NaOH + H2SO4 Na2SO4 + H2O 1. Get Yourself an unbalanced equation. 2. Draw boxes around all the chemical formulas. NaOH + H2SO4 Na2SO4 + H2O •You have 11 problems to balance. •Show Your Final Inventory Chart on the Back. •Do TWO and get them checked before you complete the page. 1. Balancing Equations – Due Tomorrow