Unit 6- Stoichiometry Day 6
advertisement

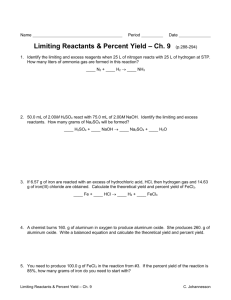
PLEASE GET OUT YOUR GREEN PACKET & RUBRIC & HW SHEET & HIGHLIGHTER Work Day! Quest Day! LIMITING REACTANTS AND PERCENT YIELD Ex: Sodium sulfate and water are created when 50 g of NaOH and 75 g of H2SO4 are mixed. Calculate the percent yield if only 25 g of sodium sulfate are made. Steps: 1. Write and balance equation 2. Calculate how much Na2SO4 can be made with 50 g NaOH 3. Calculate how much Na2SO4 can be made with 75 g of H2SO4 The lower # from 2 and 3 is how much Na2SO4 you can make! 1. Percent yield = actual yield (25g)/theoretical yield (answer above) x 100 How much did I actually get/How much should I have gotten? ANSWERS 1. H2SO4 (aq) + 2NaOH (aq) Na2SO4 (aq) + 2H2O (l) 2. (50g/40.00/2*140.15)=88.78125 90 g of Na2SO4 3. (75/98.09*140.15)=107.159 110 g of Na2SO4 NaOH is the limiting reagent so I can only make 90 grams of Na2SO4 4. 25 g / 90 g = 27.77777… 30 % yield DISCUSSION Do you think that you will have more or less copper than you started with? Why could a group have less copper? Why could a group have “more copper”? HW REVIEW Review the homework with your lab group. We will check answers as a class in a bit. Log on homework sheet and total “Unit 6 homework” Out of 40 points & highlight WORK DAY! Homework: Finish PowerPoint Finish green packet 1. Please retrieve your copper from the hood 2. Weigh your copper and filter paper in the back 3. Calculate the weight of your copper (subtract off your filter paper) 4. Tell the teacher your final mass 5. They will tell you your initial mass 6. Continue working on your PowerPoint! WORK TIME! Work on your PowerPoint as a group Finish your green packet Last 30 minutes for Quest! Homework: Finish powerpoint Finish green packet STOICHIOMETRY QUEST Homework: Finish PowerPoint Finish green packet Exit Task: (when finished with your Quest) On a piece of paper WITHOUT your name. (or with your name… who cares!) What words of advice do you have for next year’s students doing the copper lab?