Ozone/CFCs
advertisement

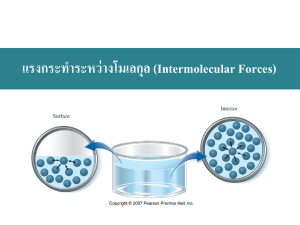
Chapter 12 – States of Matter 12.1 Gases [KMT, diffusion/effusion, pressure] 12.2 Forces of Attraction 12.3 Liquids and Solids 12.4 Phase Changes Section 12.1 Gases Gases expand, diffuse, exert pressure, and can be compressed because they are in a low density state consisting of tiny, constantly-moving particles. • Use the kinetic-molecular theory to explain the behavior of gases. • Describe how mass affects the rates of diffusion and effusion. • Explain how gas pressure is measured and calculate the partial pressure of a gas. Section 12.1 Gases Key Concepts • The kinetic-molecular theory explains the properties of gases in terms of the size, motion, and energy of their particles. • Dalton’s law of partial pressures is used to determine the pressures of individual gases in gas mixtures. • Graham’s law is used to compare the diffusion rates of two gases. Kinetic-Molecular Theory Liquids and solids have wide range of physical properties at room temperature Gases at room temperature usually display similar physical properties despite having different compositions Why? Kinetic-Molecular Theory KMT describes gas behavior by making assumptions about the size, motion, and energy of gas particles Kinetic “to move” Objects in motion have kinetic energy KE = ½ m v2 = kinetic energy of particle • m = mass, v = velocity KMT - General Explains ideal gas behavior Assumptions simplify the theory • don’t work in real gases General picture for gas particles Small - can ignore their volume In constant motion • their collisions cause pressure KMT Assumptions (1) Small particles, far apart • Gas mostly empty space • Reason for compressibility of gases No interactions between particles • No attractive or repulsive forces between molecules, except for direct collision KMT Assumptions (2) Constant straight-line motion until collision with wall or other molecule Collisions are elastic • No kinetic energy lost during collision • KE1(b) + KE2(b) = KE1(a) + KE2(a) 1,2 = particles 1 & 2 (b) = before collision (a) = after collision • KE1(b) = KE1(a) for collision with wall KMT Assumptions (3) Temperature (T) is a measure of the average kinetic energy of gas particles At a given temperature, all gases have the same average KE KE(avg) = ½ m v2 = f(T) only Raise T, KE(avg) & speed increase Lower T, KE(avg) & speed decrease KMT – Gas velocities KE(avg) = ½ m v2 = f(T) only At 25 C, H2 & N2 have same KE(avg) m(N2) / m(H2) = 28 / 2 = 14 [v2(H2)]avg / [v2(N2)]avg = 14 [v(H2)]avg = 14 [v(N2)]avg = 3.74 [v(N2)]avg Lighter gases move faster than heavier ones (at same temperature) Range of velocities Temperature is an average Molecules of many speeds in the average Shown on a graph called a velocity distribution Number of Particles 273 K 1273 K 2000 K Molecular Velocity Maxwell-Boltzmann Distribution Curve Lighter molecules move faster on average O2 - heavy Note how fast they move H2 - light Gas Molecule Velocities http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/eqpar.html#c2 http://hyperphysics.phy-astr.gsu.edu/hbase/sound/souspe3.html#c1 At 25C, vavg for H2 = 1776 m/s = 3974 mi/hr At 25C, vavg for Xe = 219 m/s = 491 mi/hr For comparison, the speed of sound in dry air at 25C vsound = 346 m/s = 776 mi/hr KMT & Gas Behavior Low density Chlorine gas 2.95 10-3 g/mL @ 20C Cl2 molecular mass = 70.9 amu Liquid water 1.0 g/mL @ 20C H2O molecular mass = 18.0 Lots of space between gas molecules relative to a liquid KMT & Gas Behavior Compression – Expansion Large amount of space allows compression Constant motion of particles explains expansion Expand Low Density Low P Compress High Density High P KMT & Diffusion/Effusion Diffusion – the movement of one material through another • One gas moves through another gas – no interactions Movement of odor molecules (perfume) Move from high to low areas of concentration Lighter gases diffuse faster KMT & Diffusion/Effusion Effusion – escape of gas through small opening Thomas Graham – various gases effusing into vacuum Graham’s Law of Effusion Rate 1/ molar mass Also can apply to diffusion rates rate A / rate B = mass B/ mass A Gas Effusion Through Pinhole Vacuum Sealed Separation With Pinhole in Separation Graham’s Law: Sample Problem 12.1 Ratio of diffusion rate of ammonia (MM=17.0 g/mol) to HCl (MM=36.5 g/mol)? rate A / rate B = (mass B / mass A) rate NH3 / rate HCl = (36.5 g/mol / 17.0 g/mol) rate NH3 / rate HCl = 1.47 Practice – Effusion/Diffusion Problems 1- 3 page 405 Problems 42 - 44 page 434 Pressure Pressure is force per unit area P= F A SI unit = pascal (Pa) = 1 N/m2 Gas molecules fill container Molecules move around and hit sides Collisions with sides provide force Container has area Measured with a barometer Atmospheric Pressure Column of air extending from sea level to the upper atmosphere Pressure – Altitude Dependence Standard atmospheric pressure refers to pressure at sea level As the altitude increases, the pressure decreases Pressure – Altitude Dependence Vacuum P exerted by Hg column 1 atm Pressure Barometer Pressure of atmosphere at sea level will hold a column of mercury (Hg) 760 mm high 1 atm = 760 mm Hg = 101.3 kPa Open end Manometer h Gas Column of mercury to measure pressure h is how much lower the pressure is than outside Manometer Open end h Gas Column of mercury to measure pressure h is how much higher the pressure is than outside Units of Pressure (1) 1 atmosphere = 1 atm (average sea level pressure at 0C) Column of mercury related units • useful for mercury barometers and manometers 1 atm = 760 mm Hg 1 mm Hg = 1 torr 1 atm = 760 torr Units of Pressure (2) Units directly in terms of pressure 1 atm = 101,325 Pascals = 101.325 kPa Pascal (Pa) = SI unit of pressure • Pa = N/m2 (force of 1 Newton per m2) 1 atm = 14.7 psi (pounds force per in2) • Common US engineering unit • We will deal primarily with SI unit Partial Pressures Each gas in a mixture exerts pressure independently of other gases present Partial Pressure (PP)– portion of the total pressure contributed by a single gas PP depends upon • # moles, volume, temperature PP independent of identity of gas Dalton’s Law of Partial Pressures Total pressure of gas mixture (Ptotal) = sum of partial pressures (Pi) Ptotal = P1 + P2 + … + Pn + 1 mol He P1 1 mol H2 P2 2 mol gas Ptotal=P1+P2 Dalton’s Law of Partial Pressures Water has a vapor pressure which depends upon temperature [f(T)] Water molecules have kinetic energy and can escape from liquid (see following slide) Energy Distribution of Molecules in a Liquid Minimum KE needed for vaporization Kinetic Energy (KE) Vapor Pressue Kinetic energy (or velocity) distribution of molecules means that a fraction of molecules have sufficient energy to overcome intermolecular forces and escape the liquid Origin of a Vapor Pressure Open Closed H2O Vapor Liquid H 2O Saturated Vapor Pressure – H2O Generating and Collecting a Gas Decomposing KClO3(s) to produce O2(g) Collecting a Gas Over Water Dalton’s Law of Partial Pressures If have a gas collected over water, total pressure given by Ptotal = Pgas + Pw Pw = vapor pressure of water at a given temperature Dalton’s Law of Partial Pressures Pw = vapor pressure of water at a given temperature • If T = 100C, then Pw=1 atm What happens to PP of N2, O2 (air) over boiling water at sea level? Dalton’s Law: Example Problem 12.2 Mixture of O2, CO2 and N2 gases has total pressure of 0.97 atm. PP of O2 if PP(CO2) = 0.70 atm and PP(N2) = 0.12 atm? Ptotal = P1 + P2 + … + Pn Ptotal = PN2 + PCO2 + PO2 PO2 = Ptotal PN2 PCO2 PO2 = 0.97 atm 0.12 atm 0.70 atm PO2 = 0.15 atm Practice (Partial Pressure, Units) Problems 4 - 7, 409 Problems 45 - 46 page 434 (Dalton’s) Problems 47- 49 page 434 (P units) Chapter 12 – States of Matter 12.1 12.2 12.3 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Section 12.2 Forces of Attraction Intermolecular forces—including dispersion forces, dipole-dipole forces, and hydrogen bonds—determine a substance’s state at a given temperature. • Describe intramolecular forces. • Compare and contrast intermolecular forces. • Identify the intermolecular forces that will act on a specific molecule. • Relate the strength of intermolecular forces to properties such as melting and boiling points. Section 12.2 Forces of Attraction Key Concepts • Intramolecular (bonding) forces are stronger than intermolecular forces. • London dispersion (aka induced dipole – induced dipole) forces are intermolecular forces between temporary dipoles. • London dispersion forces increase with molecular size and with the amount of surface contact with surrounding molecules. • Dipole-dipole forces occur between polar molecules. • For hydrogen bonding to occur, a hydrogen atom must be bonded directly to F, N, or O. • Hydrogen bonding is the strongest of the intermolecular forces. Molecular Forces Compared Forces between molecules are intermolecular forces Bond is intramolecular force Intramolecular Forces (Bonds) Intermolecular forces Inside molecules, atoms are bonded to each other • Bonds are intramolecular forces Intermolecular refers to forces between molecules • Hold molecules together in condensed states (liquid, solid) Intramolecular (Bonding) vs Intermolecular Forces Relative Strength of Forces Strong (intramolecular) • covalent bonding • ionic bonding Weak (intermolecular) • Dipole dipole • London dispersion forces During phase changes the molecules stay intact • Energy used to overcome intermolecular forces Relative Strength of Forces Intermolecular - between molecules Intramolecular – hold atoms together • 41 kJ vaporize 1 mole H2O (inter) • 930 kJ to break all O-H bonds in 1 mole of water (intra) BP, MP, VP measures of strength of intermolecular forces • Higher boiling point stronger forces Induced Dipoles - Example d+ d- H H d+ d H H Induces Instantaneous Two second nonpolar instantaneous dipole molecules created dipole London Dispersion Force Non-polar molecules also exert forces on each other • Otherwise, no solids or liquids Electrons are not evenly distributed at every instant in time Have an instantaneous dipole Induces a dipole in the atom next to it Induced dipole- induced dipole interaction London Dispersion Force Due to induced temporary dipoles • Act between all molecules • Only force between nonpolar molecules and noble gas atoms Strength depends primarily on molecular size • Very weak for small molecules • Fairly strong for large ones Molecular shape also plays a role • Spherical vs elongated molecules Dispersion Force & Molecular Size Dispersion Force – Molecular Shape n-Pentane C5H12 BP = 309.4 K Neopentane C5H12 BP = 282.7 K Dispersion Force & Molecular Shape higher boiling point weaker dispersion forces; lower boiling point Long skinny molecule … compact molecule Other Kinds of Induced Dipoles Dispersion Forces (Ar, Cl2) Ion - induced dipole Dipole – induced dipole Polarity and Dipole Moment + Dipole _ Dipole moment is a vector pointing from center of - charge to center of + charge Magnitude proportional to size of charges and to separation distance All polar covalent bonds have a dipole moment Dipole-Dipole Interactions Dipole – Dipole Interactions Orientation of Polar Molecules in a solid Dipole-Dipole Interactions The more polar the molecule, the higher the boiling point Ion – Dipole Interactions Attractive forces between an ion and a polar molecule Ion-Dipole Interactions Hydrogen Bonding Especially strong dipole-dipole forces when H is attached to F, O, or N These three because• Have high electronegativity • Are small enough to get close Effects BP & other properties affected by intermolecular forces Hydrogen Bonding in Water Each O has 2 lone pairs; interacts with two other H2Os 100 Boiling Points H 2O 0ºC HF Hydrogen bonding NH3 H2Se H2S HCl -100 PH3 SiH4 -200 CH4 AsH3 HBr GeH4 H2Te SbH3 HI SnH4 Nonpolar (tetrahedral) Properties of 3 Molecular Compounds Polar, multiple H-bonds Nonpolar -164 Polar, multiple H-bonds -33.4 Intermolecular Hydrogen Bonds Intermolecular hydrogen bonds give proteins their secondary shape, forcing protein molecules into particular orientations, like a folded sheet Intramolecular Hydrogen Bonds Intramolecular hydrogen bonds can cause proteins to take a helical shape Hydrogen Bonding in Nylon Hydrogen bonding helps make nylon strong Inter- and Intramolecular Hydrogen Bonding in Cellulose Ion-Molecule & Intermolecular Forces Summary Hydrogen Bond Ion Dipole Dipole – Induced Ion – Induced Dipole Dipole Dipole-Dipole Dispersion What type(s) of intermolecular forces exist between each of the following molecules? HBr HBr polar: dipole-dipole forces Also dispersion forces CH4 CH4 nonpolar: dispersion forces SO2 S Bent shape polar: dipole-dipole + disp. Chapter 12 – States of Matter 12.1 12.2 12.3 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Section 12.3 Liquids and Solids The particles in solids and liquids have a limited range of motion and are not easily compressed. • Contrast the arrangement of particles in liquids and solids. • Describe the factors that affect surface tension, viscosity, capillary action, beading, and the formation of a meniscus. • Distinguish between adhesion and cohesion. • Explain how the unit cell and crystal lattice are related. Section 12.3 Liquids and Solids • Explain why ice floats in water but almost all other solids sink in their liquids • Describe the factors that are different in the 7 different crystal categories. • Draw the simple, body centered, and face centered cubic unit cells. • Identify the 5 types of crystalline solids, give an example of each type and be able to rank the order of properties such as melting point among the various types. • Distinguish among crystalline, amorphous, and quasi-crystalline solids. Section 12.3 Liquids and Solids Key Concepts • The kinetic-molecular theory explains the behavior of solids and liquids. • Intermolecular forces in liquids affect viscosity, surface tension, cohesion, and adhesion. • Crystalline solids can be classified by their shape and composition. Three States of Matter Liquids – Density/Compressibility At 25°C and 1 atm pressure, liquids much denser than gases • Liquid water 1250 x denser than vapor • Both have ~ same kinetic energy Liquids not very compressible • High P needed to reduce V by a few % Liquids – Fluidity Diffusion of liquid molecules through a liquid much slower than gas through gas Liquids in this sense are “less fluid” than gases Liquids – Viscosity Measure of resistance of liquid to flow High viscosity high flow resistance • Corn syrup higher viscosity than water High viscosity high intermolecular forces • Strong hydrogen bonding Glycerol – 3 OH groups per molecule • Long chains as opposed to compact Oils – typically long carbon chains C18 Glycerol – Multiple Hydrogen Bonding Viscosity and Temperature Viscosity decreases with increasing temperature • Added kinetic energy makes it easier for molecules to overcome intermolecular forces Viscosity and Temperature Issue for motor oils • Winter need low viscosity for flow • Summer need higher viscosity to provide adequate lubrication properties Multi-grade oils have chemical additives for automatic viscosity control • Low T – compact, no strong effect • High T – uncoil, tangle with oil, increase viscosity Liquids Additional properties due to internal attraction of molecules • Surface tension • Capillary action • Beading Stronger intermolecular forces cause each of these to increase Surface Tension Definition: amount of energy required to stretch or increase surface of liquid by a unit area Strong intermolecular forces high surface tension Molecules at surface pulled toward interior Interior Surface molecules area pulled in all minimized directions Surface Tension & Detergents (Surfactants) Surfactants are compounds that lower surface tension (typically of water) – all detergents contain surfactants Typical detergent molecule: sodium lauryl sulfate – charged (polar) head & nonpolar tail Surface Tension & Detergents Surfactant forms micelle or monolayer in water Former good for removing oily dirt Latter changes surface tension Air Monolayer Bulk Water Micelle Surface Tension on a Needle Needle floats, even though density of steel much higher than density of water Needle actually rests in small depression in liquid surface Vertical components of force balance weight Cohesive vs Adhesive Forces Intermolecular forces are cohesive, connecting like things • Main cohesive force acting in liquid water is hydrogen bond Adhesive forces refer to forces between the molecule and something else, most often a solid surface Liquids in Contact with Solid Surface – Case 1 Adhesive forces greater than cohesive forces Liquid clings to walls of container Liquid “wets” surface Water Glass Liquids in Contact with Solid Surface – Case 2 Cohesive forces greater than adhesive forces Liquid curves downward Liquid does not “wet” surface Mercury Glass Capillary Action Liquids spontaneously rise in a narrow tube Glass is polar – attracts water molecule Strong adhesive forces between water and glass surface Capillary Action / Meniscus Cohesion Adhesion Adhesive Forces Capillary Action Result of surface tension and adhesive forces Liquid rises when adhesive forces greater than cohesive forces At point of contact between liquid and solid, upward forces are as shown in diagram Capillary Action Cohesive forces greater than adhesive forces Level of fluid in tube will be below surface of surrounding fluid Contact Angle Paraffin – not wetted Left: Φ > 90°; cohesive forces > adhesive forces Right: Φ < 90°; adhesive forces > cohesive forces Beading Liquid spreads; adhesive forces are comparable in strength to cohesive forces Liquid “beads up.” Which forces are stronger, adhesive or cohesive? Beading If polar substance placed on non-polar surface: • There are cohesive, • But no adhesive forces Beading - Mercury Solids KMT predicts that at same temperature, solid particles have same amount of KE as liquid • Strong forces between particles • Must be in motion – vibrations Higher degree of order in solid than in liquid Solids are not “fluid” (Exception – fluidized beds) Solids - Density Most solids more dense than their liquid phase • Typically 10% higher • Water an exception – solid structure less closely packed than liquid Thermal expansion solid-liquid-gas Density Normally, density (r) changes as solid liquid gas Temperature Thermal Expansion of Water 1.0004 Density (kg/m3) Density of ice less than water at 0C • Icebergs float Density of water is maximum at 4°C • Nearly frozen water floats to top of lake and hence freezes at surface 1.0002 1.0000 0 4 8 Temperature (°C) Hexagonal Structure of Ordinary Ice Holes in structure gives ice a density less than water Crystalline Solids Atoms regularly arranged in crystal lattice Unit cell - smallest arrangement of connected points that can be repeated in 3 dimensions to form lattice (smallest arrangement of atoms in a crystal lattice that has same symmetry as whole crystal) Unit Cell in Crystal Lattice Two Different Unit Cells in NaCl Crystal Categories (Types of Unit Cells) 7 categories based on overall shape Difference based on relative lengths of sides and angles between sides of unit cell 7 Crystal Categories - Summary 4 Types of Unit Cell (Orthorhombic Crystal Class – only one with all 4) Body Primitive Centered Face Side Centered Centered Cubic Lattice Types (Unit Cells) Simple Cubic Body-Centered Cubic Face-Centered Cubic Cubic Unit Cells Simple Cubic Body-Centered Face-Centered NaCl: Anions in FCC Unit Cell Only ions connected by lines actually touch Cl- in contact with center Na+ - 6 coordinate Unit Cell in NaCl Slice Through Atoms Cubic Unit Cells Types of Crystalline Solids (Table 12.5, p 422) ions Types of Crystalline Solids (Tab. 12.5) Atomic (noble gases only) • Ar Molecular • Ice (individual H2O molecules) Covalent network (limited category) • Diamond, quartz (SiO2), SiC,BN Ionic • NaCl Metallic • Cu (ions in lattice – book incorrect p. 422) Molecular Solids Held together by • Dispersion forces Larger for large molecules • Dipole-Dipole forces • Hydrogen Bonds Physical Properties – Molecular Solids Melting/boiling points low compared to ionic substances Many are gases or volatile liquids • O2, CO2, H2S Form relatively soft solids • Wax (typically a large hydrocarbon) Poor conductors of heat and electricity • No ions or delocalized electrons Covalent Network Solids Interconnected covalent bonds – essentially one gigantic molecule Brittle, hard, nonconductors Diamond SiO2 (quartz) SiC BN Crystal Structure of Diamond 3-dimensional network extremely strong, rigid What kind of forces must be overcome to melt diamond? Crystal Structure of Graphite Hexagons of sp2hybridized carbon (graphene) Relatively weak forces between layers Covalent Network Solids Network for graphite in-plane only Diamond Graphite Substance MP (C) Substance MP (C) Diamond 3550 Fe 1536 W 3422 Fe2O3 1460 MgO 2900 NaCovalent 890 2SO4 Network SiC 2700 NaCl Solids 808 Transition CaO 2600 CaCl2 782 Al2O3 2040 MgCl2 714 Metals Representative ZnO 1975 Al Metal 660 Metal Oxides 275 Alkali Li2O 1700 ZnCl 2 Metal SiO 1700 K 63 2 Amorphous Solids Non-crystalline solids • No regular arrangement in space Formed when material cooled quickly • Atoms don’t have time to get into ordered arrangement Glass, rubber, plastics, obsidian (lava) Quasicrystals (NIB) Although textbook lists only crystalline and amorphous solids, a third arrangement exists – quasicrystalline solids Atoms are arranged in regular patterns like in a normal crystal but the pattern never repeats itself Quasiperiodic crystal (quasicrystal) - structure that is ordered but not periodic Quasicrystalline pattern can continuously fill all available space, but it lacks translational symmetry (shifted copy will never match exactly with its original) http://en.wikipedia.org/wiki/Quasicrystal#cite_note-0 Quasicrystal Quasicrystals exist universally in many metallic alloys and some polymers. Quasicrystals are found most often in aluminium alloys but numerous other compositions are also known. http://en.wikipedia.org/wiki/File:Penrose_Tiling_%28Rhombi%29.svg Penrose Tiling Illustrates Type of Arrangement in a Quasicrystal (regular but non repeating) http://www.livescience.com/16393-nobel-prize-chemistry-quasicrystals.html Silver Aluminum Quasicrystal Regular patterns that follow mathematical rules but don't repeat themselves. CREDIT: AMES Lab, U.S. DOE Practice Problems 17-19, 21-23 page 403 Problems 43-51 page 414 Chapter 12 – States of Matter 12.1 12.2 12.3 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Section 12.4 Phase Changes Matter changes phase when energy is added or removed. • Explain how the addition and removal of energy can cause a phase change. • Name the six types of phase changes and specify if the change is endo- or exothermic. • Explain the terms superheated and supercooled as applied to liquids • Interpret vapor pressure and its dependence on temperature in terms of kinetic molecular theory and the strength of intermolecular forces. Section 12.4 Phase Changes • Interpret a phase diagram including the special points (triple and critical). • Explain how the critical temperature and pressure relate to the ability to liquefy a substance. Section 12.4 Phase Changes Key Concepts • States of a substance are referred to as phases when they coexist as physically distinct parts of a mixture. • Energy changes occur during phase changes. • Phase diagrams show how different temperatures and pressures affect the phase of a substance. Phase Changes and Energy Heat is transfer of energy from higher temperature (T2) object to lower temperature (T1) object In phase changes, energy used to overcome (disrupt) intermolecular forces between molecules For phase changes at equilibrium, T2 = T1 (both phases at same T) Phase Changes and Energy Require energy (endothermic process) • Vaporization • Sublimation • Melting Release energy (exothermic process) • Condensation • Deposition • Freezing Melting Amount of energy required to melt substance depends on strength of intermolecular forces Melting point (MP) - temperature at which solid and liquid phases coexist For amorphous solids, exact melting point more difficult to determine Supercooled Liquids Under certain conditions (generally, pure liquids that lack nucleation sites), liquids can be cooled below their freezing points and still remain liquid Called a supercooled liquid Shock or introduction of suitable nuclei can cause rapid freezing Superheated Liquids Under certain conditions (generally, pure liquids that lack nucleation sites – smooth surface cups), liquids can be heated above their boiling points and still remain liquid Called a superheated liquid Shock or introduction of suitable nuclei can cause rapid boiling Types of Crystalline Solids (Table 12.5, p 422) ions Melting & Types of Solids (Tbl 12.5) Ionic, covalent network, and many metallic solids generally have high MPs – forces involved are bonding forces Atomic solids low MPs Molecular solids low to moderate MPs • H-bonding solids requires more energy than most polar solids because of stronger dipole-dipole forces Vaporization Process by which liquid changes to gas (vapor) Kinetic energy (or velocity) distribution means that a fraction of molecules have sufficient energy to overcome intermolecular forces and escape the liquid – see following slide Energy Distribution of Molecules in a Liquid Minimum KE needed for vaporization Kinetic Energy (KE) Vaporization Vaporization occurs at surface – fewer forces acting upon a surface molecule Called evaporation Those molecules that do escape exert a gas pressure (partial pressure) that is equal to the vapor pressure • True even at temperatures near the freezing point Vapor Pressure Liquid in Closed Container Vapor Pressure – Approach to Equilbirium Rate Rate of evaporation Rate of Equal rates – equilibrium conden sation vapor pressure attained Time Measuring Vapor Pressure Before Evaporation At Equilibrium Vapor Pressure – Differing Liquids 0 torr Vacu um 24 torr H2O 65 torr 545 torr Etha nol Diethyl ether Vapor Pressure Fraction of molecules having sufficient energy to overcome intermolecular forces at 2 different temperatures Vapor Pressure Effect of Temperature Vapor Pressure – T Dependence Boiling Temperature at which vapor pressure = atmospheric pressure (normal BP) Can superheat if no nuclei present Boiling (as with melting) requires energy Temperature of liquid same as temperature of vapor produced during boiling process Sublimation Solid changes directly to gas without first becoming liquid • Iodine • Carbon dioxide • Napthalene (moth balls) • Solid air fresheners • Ice at low pressures (freeze drying) • Water in your freezer Energy-Releasing Transformations Condensation (opposite of evaporation) Deposition (opposite of sublimation) • High altitude snowflake formation Freezing (opposite of melting) Phase Diagrams Pressure vs Temperature plots with phases identified Lines on phase diagram indicate where phase changes can occur Can find MP, BP, VP as f(T), etc Triple point – all 3 phases simultaneously present • Multiple solid phases multiple triple pts. Phase Diagram Pressure Liquid Solid Gas Temperature Phase Diagram - Critical Point High pressures can liquify a gas Critical Temperature – temperature beyond which cannot liquify a vapor regardless of the pressure applied • Liquid and gas phases indistinguishable Critical Pressure – the vapor pressure at the critical temperature Critical Point – the point defined by the critical temperature and pressure Phase Diagram - Water Pressure (atm) X Temperature (C) Pressure (atm) Phase Diagram – Carbon Dioxide Temperature (C) Phase Diagram - Sulfur With 2 separate solid phases (monoclinic, rhombic), have 3 triple points The Critical Point At higher temp At room temp more vapor & its relatively little vapor density increases … at low density … while density of liquid decreases; molecular motion increases At Tc, densities of liquid & vapor equal -single phase Phase Diagram - Critical Point Critical Temperatures and Pressures These 4 gases can’t be liquefied at room temperature, regardless of applied pressure. Why not? Practice (Phase Changes) Problems 27-33 page 430 Problems 75-82 page 435 End of Chapter