Urinary System
advertisement

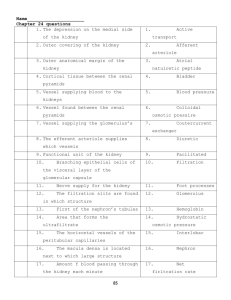
CLINICAL PHARMACY II CHP233 PATHOPHYSIOLOGY II Course Description • Provide a thorough knowledge of the pathology of various conditions that produce alteration in human physiology. • Get a baseline knowledge of its application in other subject of pharmacy such as pharmacotherapy and clinical practice. Objectives • Describe the etiology of the human diseases. • Outline the mechanisms leading to the disease. • Correlate pathophysiology to clinical manifestations in diseases. Textbooks and References 1. Pocket companion to pathologic basis of disease: by Robbins and Cotran. (7th edition). 2. Pathophysiology: clinical Concepts of Disease Process: By Sylvia Price and Lorraine Wilson. (5th edition). 3. Essentials of Pathophysiology for Pharmacy: by Martin Zdanowicz. Lectures/Practical (week) • Sunday: → 11-12 AM (1 hour) • Monday: → 8-11AM (3 hours) Course Contents 1. 2. 3. 4. 5. 6. Urinary tract disorders Gastrointestinal disorders Endocrine system disorders Musculoskeletal disorders Pulmonary disorders Neurological and psychological disorders Evaluation Quiz 1 week 6…….15 points Quiz 2 week 12 …….15 points Practical Ex. week 14……20 points Activities and attendence…….10 points Final Ex………..40 points Urinary System Dr. amal abd el moneim Gross Anatomy • Consist of six organs Kidneys (2) Ureters (2) Urinary bladder Urethra Right slightly lower than left due to space occupied by liver • Retorperitoneal (lie between the peritoneum and body wall) at the level Surface anatomy of the Kidney 12 cm 3cm 6 cm • Each kidney is bean-shaped and weight about 160g and measures 12cm long by 56cm wide and 2.5-3cm thick • Lateral surface convex, medial surface concave and has a slit called the hilum where it receives the renal artery, vein, ureter and lymphatic vessels Internal Structure of the Kidney • Cortex: the outer granular layer (containing many corpuscles) • Medulla: the inner layer that is formed of renal pyramids ending with a papilla and separated by renal columns from the cortex • Pelvis: expanded proximal ureter Renal papilla Microscopic structure of the Kidney • Each kidney contains 1.2 million functional units called nephrons • A nephron consist of two principal parts • Renal corpuscle(glomerulus) where the blood plasma is filtered • Renal tubule- processes the filtrate into urine • Renal Corpuscle contains: – Bowman’s capsule • Part of collecting system – Glomerulus • Afferent arteriole • Efferent arteriole • Renal Tubules 1. Proximal convoluted tubule 2. Loop of Henle 3. Distal convoluted tubule 4. Collecting tubule Two Types of Nephrons Cortical and Juxtamedullary Nephrons • Cortical Nephrons (85%)- nephrons close to the kidney surface • Have shorter nephron loops that dip only slightly into the outer medulla before turning back (Forming standered urine)---- the blood flow through cortex is rapid • Juxtamedullary nephron (15%)nephrons close (juxta) to the medulla • Have very long loops that extend to the apex of the renal pyramid • Responsible for maintaining the osmolality and acid-base balance (concentereted urine due to ADH) in addition to blood filteration ----- the blood flow in medulla is slow The renal corpuscle composed of glomerulus and Bowman’s capsule • Consist of a ball of capillaries called a glomerulus enclosed in a twolayered glomerular (Bowman’s) capsule • The parietal layer is a simple squamous epithelium. • The visceral layer consist of cells called podocytes wrapped around the capillaries. • The fluid that filters from the glomerular capillaries, called glomerular filtrate, collects in the capsular space between the parietal and visceral layer and then flows into the renal tubule. Juxtaglomerular (JG) Apparatus • Glomerular Filteration pass through three barriers: 1. Capillary endothelium 2. Basement membrane 3. Glomerular epithelium (visceral layer of Bawman’s capsule) slit pores between pedicles of bodocytes. • Juxtaglomerular Apparatus = Macula densa + JG cells (smooth muscle fibers from afferent arterioles). • Macula densa monitors BP through renin release, also macula densa produce erythropoietin. Blood supply to the kidney Receives 20% of the cardiac output Control of blood pressure Urine collection • From each renal papilla , collecting ducts collect the urine and released it into minor calyx then to major calyx then to renal pelvis to ureter ureter • Are muscular retroperitoneal tubes that extends from kidney to the urinary bladder at the angle Trigone. • The mucosa has transitional epithelium that is continuous with that of the pelvis and bladder • Urine stretches tube causing the muscularis ms. to contract in paristaltic waves, milking urine down to the bladder Nephrolithiasis (stone) • It occurs when the urine becomes too concentrated and substances crystallized. • The symptoms arise when stones begin to move down ureter causing an intense pain. • Kidney stones may formed in the pelvis or calyces of the kidney or in the ureter. Urinary Bladder • Retroperitoneal. • Have internal wrinkles (rugae) that permit expansion (capacity ~ 1L) • Trigone: is area at the base delinated by openings of ureters and urethera without muscle • It has internal (involuntary) and external sphincter (voluntary). • Its muscularis, called the detrusor muscle, • The mucosa has transitional epithelium Female urethra • It extended from the base of the bladder to the vestibula (3 – 5 Cm) • External urethral sphincter is voluntary at the pelvic floor. • UTIs (esp. E.coli) Male urethra Male: 18 – 20 Cm 1. Prostatic urethra: from the base of the bladder through the prostatic gland 2. Membranous urethra: between the prostatic gland and the penis base 3. Penile (spongy ) urethra: traverses penis to orifice Major kidney functions 1. Regulation of: Body fluid osmolarity and volume Electrolyte balance Acid-base balance Blood pressure 2. Excreation of: Metabolic product Foreign substances (pesticides, chemicals, ) 3. Secretion of: Erythropoitin 1,25-dihydroxy vitamin D3 (activation of vitamin D) Renin Prostaglandin Nephron Functional Mechanisms 1. Filtration (from glomeruli). 2. Reabsorption (from renal tubules). 3. Secretion (from the blood directly to the tubules). 4. Excretion (elimination from the body). Nephron function 1. Filtration: (the first step in urine • • • • formation) It is bulk transport of fluid from blood to kidney tubules ( isosmotic filtrate which is free from blood cells and protein ). Result of hydraulic pressure (i.e. increased water content of filterate) GFR = 180 L/day. The higher molecular weight of plasma constituent, the lower the rate of its filtration e.g. serum albumin and hemoglobin in comparison with glucose or Glomerular Filtration • The mechanism of filtration is Bulk Flow • Direction of movement: from glomerular capillaries to capsular space • Driving force: pressure gradient (net filtration pressure; NFP) • Types of pressure that affect the filtration: Favoring force: Capillary Blood Pressure (BP) Opposing force: blood colloid osmotic pressure (COP) and Capsule Pressure (CP). Glomerular Filtration Forces Glomerular filtration rate (GFR) • Amount of filtrate produced in the kidneys each minute is 125 mL/min. = 180 L/day. • Factors that alter filtration pressure and change GFR, include: Increased renal blood flow--------increased GFR Decreased plasma protein------increased GFR causes edema. Hemorrhage -----------------decreased capillary BP decreased GFR regulation: adjusting blood flow • GFR should be finely controlled to avoid excessive loss of fluid or decreased filtration with subsequent increased accumulation of waste products. • GFR is regulated through three mechanisms: 1. Renal autoregulation (renin -Ang. -Aldosterone) 2. Neural regulation: (sympathetic) 3. Hormonal regulation (ADH, NO, Endothellin, Prostaglandin E2 and prostacyclins) All these mechanisms adjust renal blood pressure and resulting blood flow. Measurement of GFR 1. Inulin: (it is polysaccharides from Dhalia plant) Freely filterable at the glomerulus. Does not bind to plasma proteins. Biologically inert. Non-toxic , neither synthesized nor metabolized in kidney. Neither absorbed nor secreted. Does not alter renal function. Can be accurately quantified. Low concentration are enough (10-20 mg/100 ml plasma) and taken by injection. Measurement of GFR 2- CREATININE: End product of muscle creatine metabolism Used in clinical sitting to measure GFR but less accurate than inulin method (because 10 % secreated from renal tubules). Serum Creatinine (0.6 to 1.2 mg/dL) • Creatinine is a break-down product of creatine phosphate in muscle, and • Creatinine is usually produced at a constant rate by the body (depending on muscle mass). • Is mainly filtered by the kidney, though a small amount is actively secreted. • There is no tubular reabsorption of creatinine. • If the filtering of the kidney is deficient, blood levels rise • Measuring Serum Creatinine is a simple test and it is the most commonly used indicator of renal function. • A rise in blood creatinine levels is observed only with marked damage to functioning Nephrons. • Therefore, this test is NOT suitable for detecting early stage kidney disease. • A better estimation of kidney function is given by the creatinine clearance test The Typical Reference Ranges 0.5 to 1.0 mg/dL for Women 0.7 to 1.2 mg/dL for Men • Female has less serum level of creatinine than male. • Elderly persons, on the other hand, may have less serum level of creatinine than the adult. • In patients with malnutrition, severe weight loss, and long standing illnesses the muscle mass tends to diminish over time and, therefore, their creatinine level may be lower than expected for their age. Creatinine Clearance • Creatinine clearance which represents the rate at which creatinine is removed from the body by the kidneys • Roughly approximates the GFR. • Its value is given in units of milliliters per minute. • Normal Range for male 75-125 ml/min Nephron function 2- Reabsorption: it is the process of returning filtered material ( 99 % of what filtered) to the bloodstream. May involve transport proteins. Normally glucose is totally reabsorbed. Two pathways of reabsorption: 1. Transcellular pathway (I.e. through the cell membrane) 2. Paracellular transport (i.e. through junctions between cells) Nephron function 3-Secretion: Material secreted to lumen of the kidney tubules from blood. Active transport (usually) of toxins and foreign substances (e.g. saccharine and some drugs as penicillin). Nephron function 4- Excretion: loss of fluid from the body in form of urine Amount excreted = (amount filtered + amount secreted) - amount of solute reabsorbed An overview of urine formation 1. Podocytes 2. Bawman’s space 3. Proximal tubules 4. Loop of Hennel 5. Distal tubules 6. Collecting duct. 7. Renal caylex 8. Renal pelvis 9. Ureter 10. urethera Urine concentration and dilution • Importance: • When there is excess water in the body and body fluid osmolarity is reduced; the kidney can excrete urine with an osmolarity as low as 50 mosm/Liter ( a concentration that is only about one six the osmolarity of normal extracellular fluid). • conversely, when there is a deficient of water and extracellular fluids osmolarity is high, the kidney can excrete urine with a concentration of about 1200 to 1400 mosm/Liter ( 4-5 times the osmolarity of normal extracellular fluid). Requirements for forming a concentrated or diluted urine 1. The controlled secretion of antidiuretic hormone (ADH), which regulates the permeability of the distal tubules and collecting ducts to water. 2. A high osmolarity of the renal medullary interstitial fluid, which provides the osmotic gradient necessary for water reabsorption to occur in the presence of high The Counter-Current Mechanism Produces a Hyperosmotic Renal Medullary Interstitium Hyperosmotic gradient in the renal medulla interstitium The role of ADH: There is a high osmolarity of the renal medullary interstitial fluid, which provides the osmotic gradient necessary for water reabsorption to occur. Whether the water actually leaves the collecting duct (by osmosis) is determined by the hormone ADH. Osmoreceptors by the hypothalamus detect the low level of water (high osmolarity), to send an impulse to the pituitary gland which releases ADH into the bloodstream. ADH makes the wall of the collecting duct more permeable to water. Regulation of Renin Secretion 1. Renal mechanism: Tension of the afferent artery (stretch receptor). Macula densa (stimulated by the content of Na ions in the distal convoluted tubule) 1. Nervous mechanism: sympathetic nerve 2. Humoral mechanism: E, NE, PGE2, PGI2 Micturation control Micturition, or urination, is the act of emptying the bladder. 1. As urine accumulates, distention of the bladder activates stretch receptors, which trigger spinal reflexes, resulting in storage of urine. 2. Voluntary initiation of voiding reflexes results in activation of the micturition center of the pons, which signals parasympathetic motor neurons that stimulate contraction of the detrusor muscle and relaxation of the urinary sphincters. Kidney functions that changed with age: 1. 2. 3. 4. Decline in the number of functional nephrons Reduction of GFR Reduced sensitivity to ADH. Problems with the micturation reflex. Some important facts • Kidney may sustain 90 % loss of nephrons and still not show apparent symptoms. • 2-4 % of population only have 1 kidney. ALTERATIONS OF KIDNEY STRUCTURE & FUNCTION IN DISEASE Renal failure • Renal failure or kidney failure (formerly called renal insufficiency) describes a medical condition in which the kidneys fail to adequately filter toxins and waste products from the blood. • Renal disease can be categorized either by: 1. The site of the lesion (eg, glomerulopathy vs tubulointerstitial disease) or by 2. The nature of the factors that have led to kidney disease (eg, immunologic, metabolic, infiltrative, infectious, hemodynamic, or toxic). BUT, the most appropriate classification is to first categorize the cause of the patient's renal failure as prerenal, intrarenal, or postrenal and then to subdivide each of these categories according to specific causes and anatomic locations Major causes of kidney disease 1. Prerenal disease (renal hypoperfusion) True volume depletion Gastrointestinal, renal, or sweat losses or bleeding Heart failure Hepatic cirrhosis (including the hepatorenal syndrome) Nephrotic syndrome (particularly after diuretic therapy for edema) Hypotension Nonsteroidal anti-inflammatory drugs Bilateral renal artery stenosis (particularly after therapy with an angiotensin-converting enzyme inhibitor) Major causes of kidney disease 2- Intrarenal disease Vascular disease Acute Vasculitis Malignant hypertension Thromboembolic disease Chronic Nephrosclerosis Glomerular disease Glomerulonephritis Nephrotic syndrome Tubular disease Acute Acute tubular necrosis Multiple myeloma Hypercalcemia Uric acid nephropathy Chronic Polycystic kidney disease Medullary sponge kidney Interstitial disease Acute Pyelonephritis Interstitial nephritis (usually druginduced) Chronic Pyelonephritis (due primarily to vesicoureteral reflux) Analgesic abuse Major causes of kidney disease 3- Postrenal disease Obstructive uropathy Prostatic disease Malignancy Calculi Congenital abnormalities Intrarenal disease Because intrarenal diseases leading to direct damage to the Nephron and interstitial tissues of the kidney, intrarenal causes considered to be the most important factor for the development of renal failure. Structure of the Glomerulus Glomerulonephritis (GN) (Bright’s disease) It is a group of diseases that result in inflammation and injury of the glomeruli (mainly through immunologic reaction). This diseases disrupt the capillary membrane and causes proteinuria, hematuria, oliguria, edema, hypertension and azotemia (the presence of nitrogenous waste in the blood). Normal glomerular membrane Damaged glomerular membrane Two types of immune mechanisms leading to glomerular diseases: 1. Injuries result from reaction of antibody with fixed glomerular antigen (e.g. SLE) 2. Injuries result from circulating antigen-antibody complexes that become trapped in the glomerular membrane (e.g. poststreptococcal infection). • Both leading to complement activation and leukocyte recruitment and glomerular damage. Glomerulonephritis (GN) • The main types of glomerulonephritis: 1. Nephritic syndrome: it is group of diseases that produce proliferative inflammatory responses ( i.e. increase the cellular component of the glomeruli with leukocyte infiltration) that increase the permeability of the glomerular capillary membrane leading to loss of RBCS and protein in urine. 2. Nephrotic syndrome: it is group of diseases that increase the permeability of the glomerular capillary membrane leading to massive loss of only protein in urine. Nephritic syndrome: It is characterised by hematuria with red cell cast, decreased GFR, azotemia, oliguria and hypertension. It is provoked by group of diseases that produce proliferative inflammatory responses ( i.e. increase the cellular component of the glomeruli e.g. endothelium, podocytes and mesengial cells). This inflammation leading to escape of RBCS and protein in urine. There are two types : 1.Acute proliferative GN 2.Rapidly progressive GN Acute proliferative GN Acute post-infectious glomerulonephritis • It is diffused proliferative injury caused by trapping of immuncomplex after infection with group A B-hemolytic streptococcai, staph. Infection, mumps, measles, or chicken box, HBV, HCV. • The reaction leading to proliferation in the endothelial cells of the glomeruli with leukocyte infilteration and escaping of RBCs and protein. • It occurs in children and young adult. • Clinical manifestation: by hematuria with red cell cast (cola-like urine), decreased GFR (due to loss of protein), azotemia, oliguria, edema in face and hand due to Na and water retention (aldosterone secretion)and hypertension (renin release). • Diagnosis: elevated antistreptolysin O • The condition may resolved spontaneously especially in children. Rapidly progressive (Crescentic) GN subacute GN • It is subacute severe focal proliferative inflammation in the glomeruli with formation of crescent shaped structure that obliterating the Bawman’s capsule (These are produced in part by proliferation of the parietal epithelial cells of Bowman's capsule in response to injury and in part by infiltration of monocytes and macrophages). • It is provoked by number of immunologic diseases e.g. SLE and Goodpasture’s syndrome (antibodies (IgG) formed against the basement membrane of the glomeruli and alveoli). • it is characterized by rapid and progressive loss of renal function with features of the nephritic syndrome, often with severe oliguria and (if untreated) death from renal failure within weeks to months. • Treatment: immunosuppresive drugs after plasma electrophoresis (to remove antibodies). Chronic Glomerulonephritis • Slowly progressive diffused glomerular destruction (oblitration) from long standing GN. • chronic GN develops insidiously and is discovered only late in its course, after the onset of renal insufficiency. • It is characterized by polyuria or oliguria, proteinuria, hypertension, progressive azotemia and death from uremia. • The kidneys become contracted with progressive destruction in all structures inside “end-stage kidney” Within 2-40 ys. • Hemodialysis or renal transplantation may delay the rate of disease progression. Nephrotic syndrome It is the disease which affect the integrity of the glomerular capillary membrane that increased its permeability leading to massive loss of only protein in urine (>3.5 gm/day), hypoalbuminemia, generalized edema (termed anasarca) and hyperlipidemia (reflex increase in the lipoprotein synthesis by the liver) and lipiduria. N.B. At the onset there is little or no azotemia, hematuria, or hypertension. Etiology: 1. Primary (i.e. changes in the kidney): usually occur in children focal and segmental glomerulosclerosis (FSGS minimal-change disease (MCD). membranous nephropathy membranoproliferative GN 2. Secondary (i.e. Caused by systemic diseases) e.g. diabetes, amyloidosis, and SLE. It occur mainly in adults. Minimal-Change Disease (Lipoid Nephrosis) • Also called foot-process disease. (most common nephrotic syndrome in children). • is characterized by glomeruli that have a normal appearance by light microscopy but show diffuse flattened podocyte foot processes when viewed with the electron microscope without any evidence of immunologic reaction. • Respond well to corticosteroides • Prognosis well. Focal and Segmental Glomerulosclerosis • It is characterised by sclerosing or prliferation in local area of the glomeruli due to idiopathic GN or sometimes SLE, Goodpasture’s disease. • Prognosis with drug is good (50% respond) Membranous GN • Membranous nephropathy (MN) is caused by an autoimmune response against an unknown renal antigen; it is characterized by granular subepithelial deposits of antibodies with GBM thickening and loss of foot processes but little or no inflammation. • The disease is often resistant to steroid therapy. And progress to End-stage renal failure. Membranoproliferative Glomerulonephritis • It is characterized by both deposition of immune complex under the basement membrane with hyperproleferation of glomerular cells either primarily or secondary to other disease. • The prognosis is bad “developed End-stage renal failure” . Tubulointerstitial Diseases Tubulointerstitial Diseases 1. Tubulointerstitial Nephritis : involving Acute pyelonephritis (bacterial infection) Chronic pyelonephritis (back pressure and scare formation) Drug-induced interstitial nephritis (hypersensitivity immune reaction e.g. antibiotics or PG inhibition with ischemic damage e.g. anti-inflammatory agents) 2. Acute Tubular Necrosis (ATN) Tubulointerstitial Diseases • It is a group of inflammatory diseases of the kidneys that primarily involve the interstitium and tubules. • Mainly it is due to infection so it is called (pyelonephritis) that affect the pelvis and kidney parenchyma. • If it is due to secondary disease or drug it is called Tubulointerstitial Nephritis Tubulointerstitial Nephritis 1. Acute pyelonephritis: 1. 2. 3. It is a common suppurative inflammation of the kidney and the renal pelvis, which is caused by bacterial infection (mainly E.coli, other important organisms are species of Proteus, Klebsiella, Enterobacter, and Pseudomonas). Pathogenesis: Transmission of infection in ascending order from lower UTI (most common) or from bloodstream (less common). In the presence of renal obstruction or ureterovesciular reflux (UVR), the infected urine make backward pressure on the bladder, ureter, pelvis then the kidney leading to infection of the kidney. The kidney become swollen with multiabscess on its surface, and PMNs infilteration in the renaltubules and interstatium tissue leading to its destruction and cast formation. • Risk factors for development of acute pyelonephritis: 1. 2. 3. 4. 5. 6. 7. • Urinary obstruction (stone, neoplasma, prostatic hyperplasia….) Female gender (short urethra, near anus) Pregnancy (mechanical pressure) Vesicouretral reflux Catheterization. Metabolic diseases (DM, GOUT….) Immunosuppressive drugs. It is characterized by : chills, fever, and malaise, loin pain. Urinary findings include pyuria and bacteriuria. bladder and urethral irritation (dysuria, frequency, urgency). It is diagnosed mainly by “pus cells” in urine 90% of the cases respond well to antibiotic therapy. Chronic Pyelonephritis and Reflux Nephropathy • It is a morphologic entity in which predominantly interstitial inflammation and scarring of the renal parenchyma is associated with grossly visible scarring and deformity of the pelvicalyceal system either due to prolonged obstruction or reflux infection. • The clinical manifestation may still non-obviuos until deteriorated to renal failure. Acute Tubular Necrosis (ATN) • ATN is characterized morphologically by damaged tubular epithelial cells and clinically by acute suppression of renal function. • It is the most common cause of acute renal failure. • It may be developed secondary to diseases that produced marked hypotension and shock. The pattern of ATN associated with shock is called ischemic ATN. A second pattern, called nephrotoxic ATN which is developed secondary to nephrotoxic drugs (e.g. gentamycin) or organic solvents and heavy metals. • Clinical manifestation: 1. 2. 3. The initiation phase: slight decline in urine output with a rise in serum creatinine. The maintenance phase : marked oliguria. The recovery phase: steady increase in urine volume, reaching as much as about 3 L/day until renal tubules return to its normal function again. Renovascular diseases 1. Hypertension with nephrosclerosis: • Hypertension: it is sustained elevation of the blood pressure above the acceptable level (>140/90 mmHg). • Nephrosclerosis: pathologic change in the renal blood vessels as a result of hypertension leading to chronic renal failure. • N.B.: hypertension may leading to nephrosclerosis and vice versa (i.e. Renal vascular damage may leading to secondary HTN. through renin-ang.-aldosterone system). Hypertension with nephrosclerosis: • Essential HTN may be Benign ( slowly progressive) and Malignant ( rapidly progressing HTN with end organ damage). • Therefore, nephrosclerosis may be Benign or Malignant. 1. Benign nephrosclerosis: Progressive, chronic renal damage associated with benign hypertension; characterized by hyaline arteriolosclerosis (especially afferent arterioles) and narrowing of vascular lumens with resultant cortical atrophy. 2. Malignant nephrosclerosis: Acute renal injury associated with malignant hypertension; arteries and arterioles show fibrinoid necrosis and hyperplasia of smooth muscle cells; petechial hemorrhages on the cortical surface. Renovascular diseases 2- renal artery stenosis: • It is atherosclerosis of the renal arteries causing rapidly progressing HTN. • Pathogenesis: unilateral or bilateral renal artery stenosis leading to renal ischemia, activation of renin-angiotensinaldosterone system and finely causing progressive HTN. • N.B. : 1. Unilateral renal artery stenosis leading to nephrosclerosis of the other kidney. 2. The treatment of the developed HTN with ACEIs may leading to renal failure. 3. Treatment of renal artery stenosis is mainly by surgical method. Renovascular diseases 3- Thrombotic microangiopathies: • Disorders characterized by fibrin thrombi in glomeruli and small vessels resulting in acute renal failure • CAUSES: 1. Childhood hemolytic uremic syndrome is caused by endothelial injury by an E. coli toxin; 2. Thrombotic thrombocytopenic purpura is caused by defects in von Willebrand factor leading to excessive thrombosis, with platelet consumption. Acute renal failure (ARF) “ it is an acute, reversible, rapidly decline in renal function (GFR) sufficient to increase blood levels of nitrogenous wastes (azotemia) and impair fluid and electrolyte balance”. • Persons with acute renal failure often are asymptomatic, and the condition is diagnosed by observation of elevations in blood urea nitrogen (BUN) and creatinine. • Acute renal failure, although it causes an accumulation of products normally cleared by the kidney, is a reversible process if the factors causing the condition can be corrected. Causes of Acute Renal Failure e.g. NSAID, ACEIs Pathogenesis of acute renal failure Sloughing and necrosis of tubular epithelial cells leading to obstruction and increased intraluminal pressure, which reduces glomerular filtration. Afferent arteriolar vasoconstriction, caused in part by tubuloglomular feedback, results in decreased glomerular capillary filtration pressure. Tubular injury and increased intraluminal pressure cause fluid to move from the tubular lumen into the interstitium (backleak). Ischemia or nephrotoxin Decreased renal blood flow Tubular cell damage Glomerular damage Deccreased glomerular blood flow Increased NaCL delivery to macula densa Tubular obstruction Decreased GFR Backleak of filterate Decreased glomerular ultrafilteration Clinical Course of acute renal failure • The course of acute renal failure can be divided into three phases: 1. Initiating phase (oliguric phase< 400 mL/day): which lasts hours or days, is the time from the onset of the precipitating event (e.g., ischemic phase of prerenal failure or toxin exposure) until tubular injury occurs. * It is accompanied with azotemia, fluid and electrolyte disturbance. 2. Diuretic phase (2-3 weeks): it begin when the urine output increase to more than 400 mL /day. * it is caused by osmotic diuretic effect of high level of BUN and serum creatinine. Also, impaired ability of the recovered tubules to concentrate urine which leading to loss of fluid and electrolyte. Clinical Course of acute renal failure 3- The recovery phase (after 1 year): is the period during which repair of renal tissue takes place. Its onset usually is heralded by a gradual increase in urine output and a fall in serum creatinine Treatment of acute renal failure 1. Treatment of underlying cause . 2. Continuous monitoring of fluid and electrolyte. 3. Adequate caloric intake to reduce the breakdown of protein that increase BUN. 4. Control of infection with non-nephrotoxic antibiotic. 5. Dialysis until the renal function restored. CHRONIC RENAL FAILURE “It is slowly progressive and irreversible destruction of kidney structures over a period of years” Chronic Renal Failure pathophysiology There are two theories for the development of renal failure: 1. All nephrons are diseased with different degrees, or 2. Nephrons are progressively destroyed while the remaining intact nephrons become hypertrophyed to carry the entire load of the kidney with increased the urine flow rate until a point of destruction of most nephrons with appearance of signs of kidney failure. Causes of chronic renal failure (stages) 1. Tubulointerstatial diseases e.g. chronic pyelonephritis. 2. Chronic glomerulonephritis. 3. Renovascular diseases e.g. renal artery stenosis, benign and malignant nephrosclerosis. 4. Connective tissue diseases e.g. Systemic Lupus Erythematosis. 5. Congenital renal diseases e.g. polycystic kidney diseases. 6. Metabolic disorders e.g. DM, gout, hyperparathyroidism. 7. Toxic nephropathy e.g. NSAIDs abuse. 8. Postrenal obstructive causes e.g. calculi, and tumors. Clinical Course of chronic renal failure (stages) • The progression of chronic renal failure usually occurs in four stages: 1. Diminished renal reserve, 2. Renal insufficiency, 3. Renal failure, and 4. End-stage renal disease Clinical Course of chronic renal failure (stages) 1. Diminished Renal Reserve: • Diminished renal reserve occurs when the GFR drops to approximately 50% of normal. • The serum BUN and creatinine levels still are normal, and no symptoms of impaired renal function are evident. 2. Renal Insufficiency: • Renal insufficiency represents a reduction in the GFR to 20% to 50% of normal. • During this stage, azotemia, anemia, and hypertension appear. • As nephrons are destroyed, the remaining nephrons compensate for those that are lost by filtering more solute particles and water from the blood ( polyuria). Clinical Course of chronic renal failure (stages) 3. Renal Failure: • Renal failure develops when the GFR is less than 20% of normal. • At this point, the kidneys cannot regulate volume and solute composition, and edema, metabolic acidosis, and hyperkalemia develop. 4. End-Stage Renal Disease (ESRD): • End-stage renal disease (ESRD) occurs when the GFR is less than 5% of normal. • It is characterized by a reduction in renal capillaries and scarring in the glomeruli. Atrophy and fibrosis are evident in the tubules. The mass of the kidneys usually is reduced. • At this phase of treatment with dialysis or transplantation is necessary for survival. End-Stage Renal Disease (ESRD) or uremic syndrome “i.e. urine in blood” End-Stage Renal Disease (ESRD) • End-stage renal disease (ESRD) occurs when the GFR is less than 5% of normal. • Different physiologic functions may be imbalanced: Biochemical changes 1. Metabolic acidosis: Normally, The kidneys regulate blood pH by eliminating hydrogen ions produced. This is achieved through hydrogen ion secretion, sodium and bicarbonate reabsorption, and the production and elimination of ammonia and phosphate. In chronic renal failure, these mechanisms become impaired, (i.e. phosphate and ammonia retained, while HCO3decreased) and metabolic acidosis results. With progressive renal failure, CaCO3 from bone may be used to balance the excess H+. 2. Potassium imbalance: Approximately 90% of potassium excretion is through the kidneys. In polyuric stage, hypokalemia may developed. In ESRD, hyperkalemia may be aggravated especially with metabolic acidosis (due to shift of K+ from the cells to ECF) leading to fatal arrhythmia. 3. Sodium imbalance: Sodium and water are eliminated through the kidneys. In polyuric stage, hyponatremia may developed. In ESRD, hypernatremia may be developed leading to edema, hypertension and CHF. 4. hypermagnesemia: In ESRD, hypermagnesemia may be developed especially with laxative administration (e.g. milk of magnesia). 5. azotemia: In ESRD, accumulation of nitrogenous toxins (e.g. BUN, creatinine, phenolic compounds, amines…….). 6. hyperurecemia: Hyperurecemia may developed leading to deposition of urate crystals in joints and kidney (stone formation). Genitourinary changes Polyuria followed by oliguria and finally anuria. Excessive loss of protein in urine with granular cast formation. Sterility and loss of libido for both male and female. Cardiovascular changes Hypertension, CHF (due to Na+ and water retention), pericarditis (due to accumulation of toxins) and arrhythmias (due to K+, H+ accumulation). Respiratory changes Pulmonary congestion (butterfly appearance), deep inspiration (Kussmaul’s respiration), dyspnea on exertion. Pneumonitis (lung infection). Hematologic problems Normochromic, normocytic anemia due to: o Erythropoietin defeciency. o Reduced RBCs life span due to biochemical abnormalities (hemolysis). o Excessive blood loss (due to hemodialysis) o Iron defeciency and vit. B12 defeciency (due to impaired absorption in uremic patients). o Pallor, fatigue, nosebleeds and dyspnea on exertion may developed. Hematologic problems Infection with hypothermia may developed inspite normal WBCs count; due to: o Reduced chemotaxis and delayed hypersensitivity due to toxin accumulation. o Poor nutrition. o Pulmonary edema. o Using catheters. o Using corticosteroids and immunosuppressive agents. Cutaneous problems Yellow, dry, scaly skin (due to accumulation of urinary pigments). Thin, brittle hair and nail. Uremic pruritis, not removed with treatment (due to deposition of Ca++) and sometimes uremic frost (due to urea deposition). Gastrointestinal abnormalities Anorexia, nausea and vomiting. Weight loss. Metallic taste and ammonia odour of mouth (due to splitting of urea into ammonia by saliva). Stomatitis and ulceration of small and large intestine with excessive bleeding. Infection with hepatitis C during dialysis. Dietary changes 1. Protein should be restricted to avoid azotemia. 2. Carbohydrates and fats: hyperglycemia (due to decreased tissue sensitivity to insulin), and hypertriglycridemia. Neuromuscular abnormalities 1. Central nervous system: memory defects, lethargy, confusion , coma and astrexis (flapping tremors). Dialysis may leading to brain edema and dementia. 2. Peripheral neuropathy: numbness (paresthesias) of foot and hand due to damage of myelin sheath by uremic toxins. Calcium and skeletal disorders (renal osteodystrophy) There ate three types of lesions may developed: 1. Osteomalacia or rickets (60 %): due to deficiency of bone mineralization caused by deficiency of vit. D3 (active form) and Ca++ absorption. 2. Osteitis fibrosa (30 %): due to osteoclastic resorption of bone and replacement by fibrous tissue in a localized parts (secondary hyperparathyroidism may be involved). 3. Osteosclerosis (10%): it is an alternating bands of decreased and increased bone density in the vertebral column. With finally deposition of calcium in soft tissue e,g. in the sclera of the eye. Treatment of ESRD 1. Conservative treatment. 2. Dialysis. 3. Transplantation. Diagnostic Procedures in Renal Diseases Diagnostic Procedure in Renal Diseases 1. BIOCHEMICAL METHODS. 2. MORPHOLOGIC METHODS. Biochemical Methods • Many serious renal diseases do not produce symptoms until renal function is significantly impaired. • Therefore, tests for detection of renal function is important. 1. Chemical examination of urine: A) proteinuria: * normally (< 150 mg/dl excreted in urine, mainly albumin) * in renal failure (> 150 mg/dl) * Dipstick test used for examination of urine. * grading from 0 to +4 indicate the amount of protein in urine (accurate). Disadvantages: 1. Early morning samples are more concentrated. 2. False-positive in women that has contaminated urine with vaginal secretion. 3. Screening for large MW protein only. 4. Laboratory 24-hr urine collection is more accurate. Source of urine protein (proteinuria): 1. Functional e.g. from heavy exercise or fever. 2. Overflow that is caused by overproduction of abnormal protein e.g. Bence Jones protein in multiple myeloma. 3. Glomerular e.g. in GN: that help the large, negatively charged molecules to pass freely through the glomeruli. 4. Tubular e.g. chronic pyelonephritis (reflux nephropathy) or ATN. Biochemical Methods B) Hematuria: The dipstick test is easy and accurate method for screening and follow up of occult blood in urine followed by microscopic examination of urine. Ex. UTI, stone, trauma, or GN. c) Hydrogen ion concentration: Normal urine pH (4.5 – 8.0) , but due to breakdown of food and body tissues; the urine become slightly acidic. Diurnal urine pattern means: alkaline tide (i.e. raised pH after meal) followed by acidic tide: (i.e. fall in pH during sleep due to hypoventilation) Different factors which may affect urine pH: 1. Food: protein diet causing acidic urine, while vegetable diet causing alkaline urine. 2. Metabolic acidosis: acidic urine. 3. Urinary tract infection: alkaline urine. 4. Renal tubular acidosis and hypokalemia: alkaline urine. Conditions in which the change in urine pH is useful: 1. Stones that are formed in acidic urine: e.g. calcium oxalate and urate, alkalinization of urine with NaHCO3 or ingestion of food that alkalinized urine (milk or vegetables) and high fluid intake help in dissolution of these calculi. 2. Stones that are formed in alkaline urine: e.g. calcium phosphate (triple phosphate), acidification of urine with drugs or ingestion of food that acidify urine (meat) and high fluid intake help in dissolution of these calculi. Test for urine pH (Dipstick test): Precautions: 1. Fresh urine is collected (to avoid splitting of urea into ammonia which inturn alkalinize urine). 2. Remove the strip immediately to avoid washing of the reagent. 3. Compared the strip with standard one in daylight. D) Specific gravity: 1. 2. 3. 4. 5. Urinometer is used to measure the concentration of urine (osmolality) which reflect the ability of the kidney to concentrate urine> Method: Calibrate with water at 16 C Fill the cylinder with urine. Put the urinometer. Read the measure True sp.gr. Corrected for the temperature (i.e. add 0.001 for each temp. above 3C OR subtract 0.001 for each temp. below 3C) There is a specific correlation between sp,gr. and osmolality Normal urine sp.gr.= 1.010 which increased by concentration of urine an decreased by dilution of it. Glucose increase the sp.gr. While urea decrease it. Biochemical Methods 2. Glomerular filtration rate: • Less accurate method than inulin in detection of renal function but more safe. A) Creatinine clearance test: End product of tissue catabolism which is excreted mainly via glomeruli and not reabsorbed. Serum creatinine level= 0.7-1.5 mg/dl (male>female). Method: • Collect 24-hr urine and detect creatinine. • Take 1 serum sample of blood during 24-hr. and detect serum creatinine. • Calculate creatinine clearance= UxV/P B) Serum creatinine and BUN: Increased their level indicate decreased GFR (azotemia). Serum creatinine is more accurate “ constant ms mass” than BUN “ affected by dprotein in diet and endogenous protein catabolism” 3. Tubular function test: The main function of renal tubules are reabsorption and secretion which is controlled by hormones and concentration gradient. For measurement of proximal tubular function: Phenolsulfophthalein excretion test (PSP). Para-aminohipurate excretion test (PAH). For measurement of distal tubular function: Concentration & dilution test, acidification and Na conservation test. A) PSP excretion test: It is non-toxic dye excreted mainly via tubules. Method: 1.The patient should ingest 3 cups of water 30’ before injection of dye. 2.The patient should empty the bladder 15’ after injection. 3.Collect the urine in 1 L volumetric flask then add 5 ml NaOH 4.The pink color developed compared with standard. 5.Normally, the intensity of the color indicate the amount of dye eliminated. B) PAH excretion test: It is substance that is filtered by GFR and secreted through tubules. It is indicator of the renal plasma flow if it is eliminated completely. C) Concentration and dilution of urine: concentration urine test: (by measurment of sp.gr. With water 1. 2. 3. restriction) Method: Eat normal diet and restrict water after 6 PM. (diuretic should be avoided). Collect urine at the next 6,7,8 AM. One specimen should have sp.gr.> 1.003. dilution urine test: 1. 2. 3. Drink 1 L water over 30’ . Collect the urine in the next 3 hr. One specimen should have sp.gr. < 1.003. Disadvantages; 1. Nausea and vomiting may interfere with the dilution test. 2. The ability to concentrate or dilute urine lost with kidney diseases, hepatic diseases and CHF. D) Urinary acidification test: Used to measure the amount of acid eliminated via kidney. Used for diagnosis of acute tubular acidosis. Method: 1. Collect urine for 2 days. 2. NH4CL taken orally for 3 days with continuous urine collection. 3. Continuous measurement of pH within 5 days. 4. NH4+ splitted in the body and liberate H+ which eliminated through the tubules. 5. In renal tubular acidosis: the tubules failed to eliminate the acid load (pH > 5.3) E) Na conservation test: Urine is normaly Na+ free. In renal failure (chronic pyelonephritis): loss of Na+ and water decrease the plasma volume, decrease GFR) It is used to evaluate the amount of salt required to be taken or restricted in diet. Morphologic Methods 1. Microscopic examination of urine: The freshly collected urine, centrifuged and the deposits suspended in 0.5 ml urine and examined microscopically. The normal deposits: contained few cast from vagina, urinary tract, few RBCs, few WBCs. The abnormal deposits are: bacteria, casts, RBCs, WBCs. Casts indicate damage of the tubular cells. Different casts: red cell casts (GN), white cell casts (pyelonephritis), fatty casts (nephrotic syndrome), granular casts (ESRD). Cylindruria: excessive cast production which indicate renal diseases. Morphologic Methods 2- Bacteriologic examination of urine; Normally urine is sterile. Bacteria is detected in UTI. Method: 1. Collect the midstream urine after washing the external genitalia. 2. Within 30’ make 2 tests: Culture and sensitivity test: inoculation of urine into agar plate and incubated for 24 hr then count the colonies and the specific antibiotic. • Urinary test strip: detect nitrite developed with Gm –ve infection. 3- Radiologic examination: A) Intravenous pyelogram: 1. Plain radiogram of the abdomen is taken. 2. IV injection of contrast media. 3. Radiograph is taken at: 5’ for visualization of cortical lesions 15’ for visualization of calyx, pelvis, ureter abnormality 45’ for visualization of bladder abnormalities. 4. The test is C.I. in ESRD 5. Advantages: • detect the shape, size and position of the kidney • Detect the kidney ability to concentrate or dilute dye. • Detect any disease or abnormalities. 6. Retrograde pyelogram can be used to ensure the results. B) Renal ultrasonography: It depends on the reflection of ultrasound applied on the abdomen using sonogram. Uses: 1. To distinguish solid tumor from fluid-filled cyst. 2. Used in ESRD when IVP is contraindicated. 3. Detect the accurate kidney size, and abnormalities. 4. Evaluation of any complication to renal transplantation. 5. Help in introduction of needle in renal biopsy. C) Renal radionuclide imaging: injection of radioactive material then use gamma camera to detect the lesions. E.g. renovascular diseases. D) Computed tomography (CT): Can be used to visualized all urinary system and detect lesions e.g. stones, neoplasm, thrombosis using ionized radiation. E) Magnetic resonance imaging (MRI): More accurate and detailed method without application of ionized radiation (as in CT) or radiocontrast media (as in pyelogram). F) Renal arteriogram: Catheter is introduced from the femoral artery to the abdominal aorta to renal artery with injection of the contrast media. Used for detection of arterial stenosis, kidney patchy necrosis in chronic pyelonephritis. But the risk of hypersensitivity may developed. G) Renal biopsy: The patient lies on abdominal sandbag and help the kidney to protruded backward. Using needle, specimen is collected and examined microscopically for any diffused renal diseases This technique is accompanied with life-threatening bleeding.