Strong and weak acids and bases
advertisement
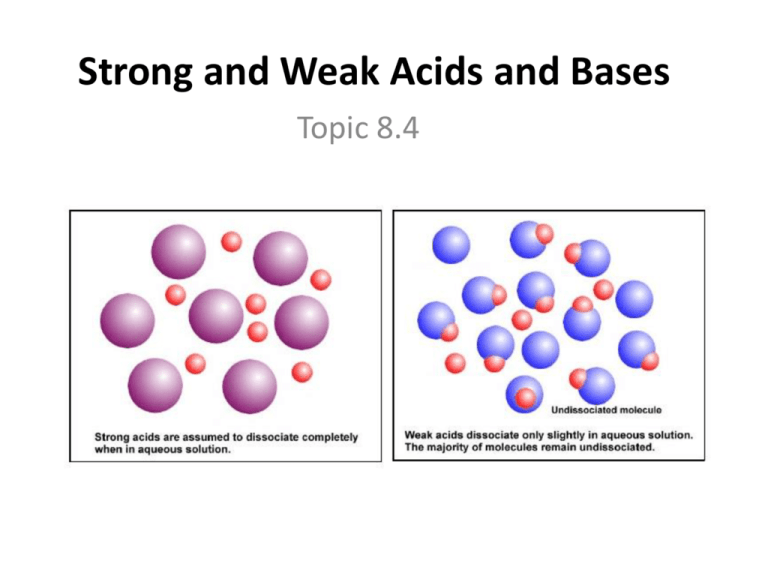
Strong and Weak Acids and Bases Topic 8.4 ACIDS • when a STRONG acid dissolves- all, or nearly all, of the acid molecules dissociate to produce H+ or H3O+ ions (reaction goes to “completion”) – have a very high Kc value • HA H+(aq) + A-(aq) 0% ~100% • HA + H2O(l) H3O+(aq) + A-(aq) 0% ~100% – equilibrium is so far to the right for strong acids that we use a yields symbol () instead of an equilibrium symbol (⇌) Initial amount of HA HA H+ A- At Equilibrium common strong acids (need to know) • hydrochloric acid: – HCl(g) + H2O(l) H3O+(aq) + Cl-(aq) • nitric acid: – HNO3(l) + H2O(l) H3O+(aq) + NO3-(aq) • sulfuric acid: – H2SO4(l) + H2O(l) H3O+(aq) + HSO4-(aq) • when a WEAK acid dissolves, very few acid molecules dissociate to produce H+ ions (equilibrium lies to the left) – have a very low Kc value • HA ⇌ H+(aq) + A-(aq) 99% ~1% • HA + H2O(l) ⇌ H3O+(aq) + A-(aq) 99% ~1% Initial amount of HA HA HA H + A- At Equilibrium common weak acids (know these) • carboxylic acids (contains one or more carboxyl groups, COOH): – methanoic acid (CHOOH) – ethanoic (acetic) acid • CH3COOH(l) + H2O(l) ⇌ CH3COO-(aq) + H3O+ – carbonic acid: (CO2 in water) • CO2(aq) + H2O(l) ⇌ H2CO3-(aq) Characteristics of strong vs. weak acids • acid strength does not change when a solution is diluted, only the concentration (molarity) or pH does • for example, HCl is always a strong acid – it just may be diluted down and therefore have a pH close to 7 and low concentration • DO NOT confuse strong and weak, with dilute and concentrated – concentrated = high molarity (M) same concentration, different strength because the week acid (CH3COOH) does not dissociate into H+ as much as the HCl does 0.1 mol dm-3 HCl(aq) 0.1 mol dm-3 0.1 mol dm-3 CH3COOH(aq) ~0.0013 mol dm-3 pH Conductivity Reaction Rate with Mg 1.00 High Fast 2.87 Low Slow Reaction Rate with CaCO3 Fast Slow [H+(aq)] – strong acids • have more H+ ions, hence a low pH • higher conductivity • react more vigorously with metals, metal oxides, metal carbonates and bicarbonates • have a more (-)∆H of neutralization (more heat) • dangerous – weak acids • opposite of strong acids BASES • when a STRONG base dissolves, nearly all the base molecules dissociate to produce hydroxide (OH-) ions solution – have a very high Kc value • BOH + (aq) OH-(aq) + B+(aq) 0% ~100% – equilibrium is so far to the right for strong bases that we use a yields symbol () instead of an equilibrium symbol (⇌) common strong bases (know these) – all group I hydroxides and barium (group 2) hydroxide • NaOH(s) + (aq) Na+(aq) + OH-(aq) • KOH(s) + (aq) K+(aq) + OH-(aq) • Ba(OH)2(aq) + (aq) Ba2+(aq) + 2OH-(aq) • when a WEAK base dissolves, very few base molecules dissociate to produce OH- ions – have a very low Kc value • BOH + (aq) ⇌ OH-(aq) + B+(aq) 99% ~1% common weak bases • ammonia – NH3(g) + (aq) ⇌ NH4+(aq) + OH-(aq) • amines (contain NH1,2, or 3) : – ethylamine • C2H5NH2(g) + H2O(l) ⇌ C2H5NH3+(aq) + OH-(aq) = group 1 on periodic table = group 2 on periodic table Strong Bases LiOH NaOH KOH RbOH CsOH Ba(OH)2 Weak Bases NH3 Amines (example: C2H5NH2) strong vs. weak base characteristics – strong bases • has more OH- ions, hence a high pH • higher conductivity • have a more (-)∆H of neutralization (more heat) • dangerous – weak bases • opposite of strong bases Using experimental data to determine acids and bases • Given the following are 0.1 M solutions, determine whether an acid or base, and strength – – – – – – – – – ph of 1: strong acid poor conductor with pH of 6: weak acid fast reaction with magnesium: strong acid [H+] = 10 -10M: weak base [H+] = 10 -4M: weak acid neutralized 0.1M HCl quickly: strong base good conductor with pH of 13: strong base slow reaction with calcium: weak acid has a high -ΔH when reacting with a base: strong acid • Strong acid Weak conjugate base – ALL of the acid donates H+ – almost NO H+ is accepted back • Weak acid Strong conjugate base • Strong base Weak conjugate acid • Weak base Strong conjugate acid Acid Strength Base Strength H2SO4 Very Strong HSO4- Very Weak HCl Cl- HNO3 NO3- H3O+ Fairly Strong H2 O HSO4- SO42- CH3COOH CH3COO- H2CO3 Weak NH4+ HCO3H2 O HCO3- Weak Less Weak NH3 Very Weak CO32OH- Fairly Strong