02A naming alkane alkene alkyne 2
advertisement

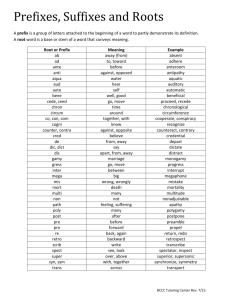
IUPAC Nomenclature of Hydrocarbons I nternational U nion of P ure and A pplied C hemistry Models Used to Represent Structures Model Example Empirical molecular formula C7H16 Expanded molecular formula CH3(CH2)5CH3 Structural formula Condensed structural formula Line structural formula CH3-CH2-CH2-CH2-CH2-CH2-CH3 Naming Alkanes • The IUPAC name of any organic compound has three basic parts Root- denotes number of carbon atoms in the longest continuous chain of carbon atoms Prefix- gives positions and names of any branches Suffix- indicates series to which the molecule belongs (e.g. alkane, alkene alkyne) Root and Side Group Names of Alkanes Number of Carbon Atoms Root Name Prefix (Side Group) 1 meth- methyl- 2 eth- ethyl- 3 prop- propyl- 4 but- butyl- 5 pent- pentyl- 6 hex- hexyl- 7 hept- heptyl- 8 oct- 9 non- 10 dec- The alkanes (CnH2n+2) C H 4 M ethane C H C H C H C H C H C H 3 2 2 2 2 3 (C H 4) C H C H 3 3 Ethane (C 2H 6) (C 3H 8) (C 4H 10) C H C H C H C H C H 3 2 2 2 3 Pentane H eptane (C 7H 16) C H C H C H C H C H C H C H C H 3 2 2 2 2 2 2 3 C H C H C H C H 3 2 2 3 B utane (C 6H 14) C H C H C H C H C H C H C H 3 2 2 2 2 2 3 C H C H C H 3 2 3 Propane H exane (C 5H 12) O ctane (C 8H 18) C H C H C H C H C H C H C H C H C H 3 2 2 2 2 2 2 2 3 N onane (C 9H 20) C H C H C H C H C H C H C H C H C H C H 3 2 2 2 2 2 2 2 2 3 D ecane (C 10H 22) Steps for Naming Alkanes 1. Identify the root • Identify the longest continuous chain • Find the root name for the number of carbons in the chain 2. Identify the suffix • Longest chain has 6 carbon atoms • Root name is hex• Compound is alkane • Suffix is -ane 1. Identify the prefix (name the branches) • Identify the number of carbon atoms in each branch • Determine the name of each branch • Number the C atoms on the longest chain to produce the lowest number combinations (or sum) for the branches • Two branches have one carbon atom and 3rd branch has 2 carbon atoms • Methyl- and ethyl- • Precede the name of each branch with the number of the carbon atom to which it is attached on the main chain. • For more than one of the same branch use a prefix: di = 2 ,tri = 3, tetra = 4, penta = 5 • Separate numbers using commas • Separate numbers and words using hyphens • 3-ethyl • 2,4-dimethyl • Arrange branches alphabetically (ignore prefixes di, tri, etc…) 4. Name the compound • Combine prefix, root, and suffix to name the compound • Prefix: 3-ethyl-2,4dimethyl • Root: hex• Suffix: -ane • Name: 3-ethyl-2,4dimethylhexane Finding the longest continuous chain of carbon atoms is not always simple all possibilites must be examined C-C C-C-C-C-C-C-C-C-C C it won’t always be the horizontal one as shown here 9 try these also …….. C-C C-C C- C-C-C-C-C-C-C-C C 6 C-C-C- C-C-C-C-C-C C 8 Name this alkane 4 3 2 1 C H C H C H C H 3 2 3 C H 3 2-methylbutane Find the longest continuous carbon chain 1 2 3 C H H HC H 3C 2C 3 C H C H 2 3 4 3-methylpentane 5 Name the Following Compound 4 3 2 1 C H C H C H C H C H C H 3 2 2 2 3 C H C H C H 2 2 3 5 6 7 4-ethylheptane Number from the end nearest the first substituent C H C H 2 3 C H C H C H C H C H C H C H 3 2 2 2 3 7 6 5 4 3 2 C H 3 4-ethyl-3-methylheptane 1 Number from the end nearest the first substituent C H 3 C H C H C H C H C H C H C H C H 3 2 2 2 2 3 8 7 6 5 4 3 2 1 C H C H 2 3 3-ethyl-5-methyloctane Use “di-” with two substituents CH3 CH3 CH CH CH3 1 2 3 4 CH3 2,3-dimethylbutane Every substituent must get a number H C 3 H C H C H C C H C H C 3 2 2 2 3 1 2 3 4 5 H C 3 3,3-dimethylhexane 6 You need numbers, even though it appears on the same carbon! C H 3 C H 3 C H HC H H 3C 2C C 3 5 4 3 2 1 C H 3 2,2,4-trimethylpentane Name the following compound C H 3 C H C H C H C H C H C H C H C H C H C H 3 2 2 2 2 2 3 1 2 3 4 C H 3 5 6 7 8 9 C H 3 3,4,8-trimethyldecane 10 Name the following compound Dimethyl alphabetized as methyl, not dimethyl C H 3 C H C H C H C H C H C H C H C H 3 2 2 2 3 1 2 3 4 C H 3 5 6 7 8 C H C H 2 3 6-ethyl-3,4-dimethyloctane Drawing Alkanes 1. Identify the root • Gives the number of carbon atoms in the main chain 1. Identify the suffix • Indicates the type of bond between carbon atoms 3. Draw and number main chain e.g. draw 3-ethyl-3-methylpentane root: pentsuffix: -ane C-C-C-C-C 1 2 3 4 5 1. Identify the prefix and draw the side groups 2. Complete the condensed structural formula Prefix: 3-ethyl-3methyl(ethyl and methyl group on carbon 3) Draw the structural diagram of 2methylheptane Physical Properties of Alkanes • Since alkanes are non-polar, they are not soluble in water Number of carbon atoms Boiling point range(oC) Uses 1 to 4 Below 30 (gases) Fuels to heat homes and cook 5 to 16 30 to 275 (liquids) automotive, diesel and jet engine fuels 16 to 22 Over 250 (heavy liquid) furnace oil Over 18 Over 400 (Semi-solid) paraffin waxes to make candles Over 26 Over 500 (Solid residues) asphalts and tars in paving Naming Alkenes 1. Identify the root • Identify longest chain with a double bond. • Assign root name based on number of carbons 1. Identify the suffix • Number the main chain by starting at the end of the chain nearest the double bond. Root: pentSuffix: -2-ene • (indicate the number of the carbon atom that precedes the double bond, only if alkene has 4 or more carbons) 1. Identify the prefix • Name the side groups on alkenes as you would for alkanes 1. Name the compound. Root: pent• Combine the prefix, Prefix: 2,3-dimethylroot and suffix. Suffix: -2-ene Name: 2,3-dimethylpent-2-ene C H H 2C 2 ethene C H H HC H 3C 2C 2 but-1-ene C H HC H 3C 2 propene C H HC HC H 3C 3 but-2-ene CH3 C CH CH3 CH3 2-methylbut-2-ene H C 3 H H H H H H H C C C C C C C 3 2 2 3 6-methylhept-2-ene C H 3 C H H HC H H 3C 2C 2C 2 C C H H H 3C 2C 2 H C C H 3 trans-6-methyl-3-propyloct-2-ene Drawing Alkenes draw a structural formula for 2-methylbut-1-ene • Identify the root • Identify the suffix • Draw and number the main carbon chain • Identify the prefix and draw the branches • Complete the structural formula by adding hydrogens Root: but- (4 carbons) Suffix: double bond between c-1 and c-2 Prefix: methyl group on c-2 Naming Alkynes 1. Identify the root • Identify longest chain with a triple bond. • Assign root name based on number of carbons 1. Identify the suffix • Number the main chain by starting at the end of the chain nearest the triple bond. Root: pentSuffix: -1-yne • (indicate the number of the carbon atom that precedes the triple bond, only if alkyne has 4 or more carbons) 1. Identify the prefix • Name the side groups on alkynes as you would for alkanes 1. Name the compound. Root: pent• Combine the prefix, Prefix: 3-methylroot and suffix. Suffix: -1-yne Name:3-methylpent-1yne Drawing Alkynes draw a structural formula for 3-ethyhex-1-yne • Identify the root • Identify the suffix • Draw and number the main chain • Identify the prefix and draw any side groups • Add enough hydrogen atoms to give each carbon atom four bonds. • Root: hex- (6 carbons) • Suffix: -1-yne (triple bond between c1and c2 • Prefix: ethyl group on c3 H C C H ethyne “acetylene” C H H H 3C 2C C but-1-yne C H 3C C H propyne C H H 3C C C 3 but-2-yne ALKYNES ( -YNE ) The functional group has precedence in numbering. CH3 C C CH2CH2CH3 Hex-2-yne functional group CH3 C C CH CH3 CH3 4-methylpent-2-yne The suffix has precedence over any branches nomenclature of halides and nitro compounds F C l flu o ro N O 2 n itro c h lo ro B r b ro m o I io d o C H 3 4-chloro-4-methylpent-2-yne C H H 3C C C C 3 C l C H 3 B r C H HC C C HC H H 3C 2C 3 5-bromo-2-methylhept-3-yne ene vs. yne: which one wins? Number from the end closest to either the double bond or the triple bond, whichever is closest to the end. Compounds are named: en-yne. 8 7 6 CH3-CH2-C 5 4 3 2 1 C-CH2-CH=CH-CH3 oct-2-en-5-yne cycloalkanes • The names of the cycloalkanes always contain the prefix cyclo • Cycloalkanes have the general formula CnH2n Cyclic molecules H H C H C H 2 H C C H H C H C H 2 2 H H C H H C y c lo p ro p a n e H H H C C H C H C H 2 2 H C C H C H C H 2 2 H H C y c lo b u ta n e C C C C H H H H H H H H H C C C H C H 2 2 C y c lo p e n ta n e H C C C H H H H C H 2 C H C H 2 2 C H 2 C H C H 2 2 H H C H C H 2 2 C C H 2 H H C y c lo h e x a n e Naming Cyclic Hydrocarbons 1. Identify the root • Determine number of carbon atoms in the ring. Assign root name 2. Identify the suffix • Not necessary to indicate location of double or triple bonds, always between c1-c2 Root: pentSuffix: -1-yne • (indicate the number of the carbon atom that precedes the triple bond, only if alkyne has 4 or more carbons) Naming Cyclic Hydrocarbons 3. Identify the prefix. • If there is only one substituent, do not use the “1”. • If there is more than one substituent, you must use all numbers, including “1”! • If there are two or more side groups, the numbering must start with a side group and then proceed in the direction that gives the lowest possible numbers to all the side groups. • If numbers are the same for two or more side groups, the side group that comes first alphabetically is assigned as c1. Naming Cyclic Hydrocarbons • If the molecule is a cyclic alkene or cyclic alkyne, the multiple bond takes highest priority. The carbon atom on one side of the multiple bonds is c-1 and one the other side is c-2. If there are side groups, the numbering starts in the position that will make the number of the carbon atoms bonded to the side groups as small as possible. 4. Name the compound • Combine the prefix, root, and suffix to name the compound. Naming Cyclic Hydrocarbons 1. Root: cyclopent 2. Suffix:-ene 3. Prefix:3,4-dimethyl Name: 3,4-dimethylcyclopentene CH3 CH3 1,1-dimethylcyclohexane C H 3 C H 3 C H H 3 C 2 4-ethyl-1,1-dimethylcyclohexane Some cycloalkanes 1,3-dimethylcyclopentane CH3 1 2 3 CH3 CH3 CH3 Drawn differently but same name. 1 = 2 3 CH3 CH3 4 3 1 2 1 CH2CH3 1-ethyl-4-methylcyclohexane E before M CH3CH2 3 CH3 2 3-ethyl-1,1-dimethylcyclobutane The more substituted carbon takes precedence even though E comes before M. Numbering starts at the most highly-substituted carbon Cl CH3 CH3 2 1 3 7 4 6 5 CH3 2-chloro-1,1,6-trimethylcycloheptane cycloalkyl groups cyclopropyl cyclopentyl cyclobutyl cyclohexyl CH3 CH2 C CH2 CH3 CH3 3-cyclobutyl-3-methylpentane Another name of a group o r o rC H 6 5 P h e n y l CH3 CH3 CH2 CH CH CH3 3-methyl-2-phenylpentane