Hsc year 12 pH exp
advertisement

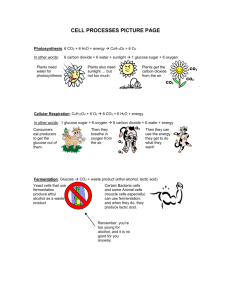
HSC YEAR 12 WYONG TAFE COMPULSORY PRACTICAL ACTIVITY THE EFFECT OF DISSOLVED CARBON DIOXIDE ON THE PH OF WATER Learning outcomes covered by this activity P2 - carry out a firsthand investigation P6 - explain how cells contribute to macro processes in organisms P11 - identify and plan improvements to investigation plans P12 - discuss the validity and reliability of data P13 - identifies appropriate terminology an reporting styles to communicate information and understanding in biology P14 - draw valid conclusions from gathered data and information P15 - implements strategies to work effectively as an individual or as a team member H12 - evaluates ways in which accuracy and reliability could be improved in investigations H13 - uses terminology and reporting styles appropriately and successfully to communicate information and understanding H14 - assess the validity of conclusions from gathered data and information THE ACTIVITY You are to plan an experiment which demonstrates the effect dissolved carbon dioxide on the pH of water. You are to choose the equipment for the experiment Carry out the experiment Record, interpret and use the data to form conclusions MATERIALS Solid calcium carbonate Dilute Hydrochloric Acid Distilled water 100mL measuring cylinder Test-tubes, one test-tube fitted with a cork and bent glass tubing as shown Test-tube rack Clamps and retort stands Plastic bag with rubber band to close it- a small freezer bag would also be suitable Universal indicator paper and a card to check to pH Lime water solution (calcium hydroxide) Page 1 of 3 METHOD 1. Choose the equipment from the materials and equipment provided and design an experiment to show the effect of CO2 on pH of distilled water. You must demonstrate the effect of CO2 from two different sources of CO2 on the pH of water. 2. Write down your experimental design and check it with your teacher. 3. Perform the experiment and record the data. 4. Try increasing the amount of CO2 and check it there is a further change in pH. 5. Interpret your data to make a conclusion about the answer. 6. Answer the questions. QUESTIONS What was the effect of CO2 on the pH of distilled water? How does pH relate to hydrogen ion concentration? From your knowledge of biology, explain how carbon dioxide changes the pH. What sources of CO2 did you use? Which of these sources is relevant to body physiology? Explain how it is made in the body. Would the change in pH be dangerous for the body? Explain If so, how does the body solve the problem? What essential measurement did you have to make before testing the effect of CO2 on the water? Why? 9. Having designed and performed this experiment, what do you think are the key points about it – assume you are explaining it to another student who has not done it before. 1. 2. 3. 4. 5. 6. 7. 8. Page 2 of 3 MARKING SCALE Your experiment will be marked on the following scale TOPIC P6, H13 P2 P2, P13, P15, H13 P12, P14, H13 H13, H14 P15, H12, H13 MARK Background information on the macroprocesses in organisms Experimental design- a diagram of the experimental set up Experimental write up with detailed explanation of the how the experiment was carried out with aim, method, equipment used, results clearly recorded and a discussion of the results. Draw conclusions from recorded results. Answer the questions by selecting and using appropriate methods to acknowledge sources of information Evaluate the accuracy and reliability of the results Identify trends, patterns and relationships as well as contradictions in data and information Identify and explain hoe data supports or refutes an Identify ways to improve the investigation TOTAL MARK AWARDED 4 1 5 4 4 2 20 Page 3 of 3