Chemistry 59-330 Lecture 5
advertisement

Vibrational Spectroscopy
A rough definition of spectroscopy is “the study of the interaction of matter
with energy (radiation in the electromagnetic spectrum).” A molecular
vibration is a periodic distortion of a molecule from its equilibrium geometry.
The energy required for a molecule to vibrate is quantized (not continuous)
and is generally in the infrared region of the electromagnetic spectrum.
DAB
For a diatomic molecule (A-B), the bond between the two
atoms can be approximated by a spring that restores the
distance between A and B to its equilibrium value. The
bond can be assigned a force constant, k (in Nm-1; the
stronger the bond, the larger k) and the relationship
between the frequency of the vibration, , is given by the
relationship:
0
rAB
k
or, more typically
2c
k
where , c is the speed of light, is the frequency in “wave
numbers” (cm-1) and is the reduced mass (in amu) of A
and B given by the equation:
m m
re
re = equilibrium distance between A and B
A
B
mA mB
DAB = energy required to dissociate into A and B atoms
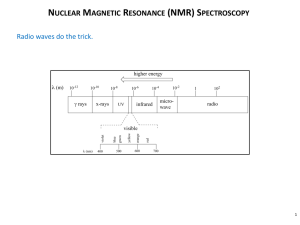
Infrared Radiation
Portion of the electromagnetic spectrum
between visible light and microwaves
full range for IR is 10000-400 cm-1
of importance here is 4000-400 cm-1 (wavenumbers)
or 2.2-25 m (wavelength)
Note: cm-1 is proportional to Energy
cm-1 = 104/m
this energy is absorbed by molecules and converted
to molecular vibration
IR Absorption
IR absorptions are characteristic of entire molecule
or essentially a molecular fingerprint
vibration spectrum appear as bands molecular
vibration is not a single energy as also depends on
molecular rotation
band intensities expressed as either transmission (T)
or absorption (A)
A = log10(1/T)
Molecular Vibrations
Stretching is a rhythmical movement along a bond
Bending is a vibration that may consist of a change
in bond angle (twisting, rocking and torsional vib.)
Vibrations that result in change of dipole moment
give rise to IR absorptions
alternating electric field produced by changing dipole
couples the molecular vibration to the oscillating
electric field of the radiation
Vibrations for H2O and CO2
3650 cm-1
3756 cm-1
1596 cm-1
Symmetrical
stretch
asymmetrical
stretch
scissoring
(inactive in IR)
for CO2
1340 cm-1
2350 cm-1
+ - +
666 cm-1
Bending for CH2
+
+
Asymmetric symmetric
stretch
stretch
2926 cm-1
2853 cm-1
Out-of plane
bend or twist
1350-1150 cm-1
+
in-plane
bend
1465 cm-1
out-of-plane
bend
1350-1150 cm-1
-
In plane bend
or rocking
1350-1150 cm-1
Assignments of Bands
For a stretching frequency interruption based on
Hooke’s Law: Frequency = 1/2c[(k/(MxMy/Mx+My)]1/2
where f = force constant of bond and M is mass
f is about 5 x105 dyne/cm for single bond
2x that for double bond and 3x that for triple bond
C-H stretch:calc: 3040 cm-1 actual CH3: 2960-2850 cm-1
Note: for C-D: stretch is 21/2 x that of C-H
Instrumentation
Requirements: source of IR radiation, sample, detector
IR Spectrophotometer
Sample Handling
IR spectra can be obtained for gases, liquids and solids
Liquids: may be neat or in solution
Neat: between to NaCl plates (0.01 mm film)
(NaCl does not absorb until 600 cm-1)
thick samples absorb too strongly: poor spectrum
Solution: cells are 0.1-1 mm thick(0.1-1 mL in volume)
requires second cell of pure solvent to correct for
absorptions of solvent
Solids: usually as a mull (supension) in nujol oil
(free of IR absorptions 4000-250 cm-1
or dispersed in KCl pellet
Spectral Interpretation
Precise and complete interpretation is NOT possible
thus must use IR in conjunction with other techniques
but
Functional group region: 4000-1300 cm-1:
eg: OH, NH, C=O, S-H, CC
Many functional groups exhibit characteristic bands
Fingerprint regions:
1300-650 cm-1absorptions here are usually complex
some interpretation is possible
similar compounds give similar spectra but fingerprint is
unique
Organic Functional Groups
An Organic Example
CN stretch
2226
Aromatic C-H bands
Nuclear Magnetic Resonance
Sample in a magnetic field absorbs radio frequency
radiation
absorption depends on certain nuclei in molecule
initially we deal with 1H (proton) NMR
inspection of NMR provides much more structural data
than MS or IR
Magnetic Nuclei
Nuclei with odd mass, odd atomic number or
both have quantized spin angular momentum
eg 11H, 21H, 136C, 147N, 3115P
spin quantum number, I = 0, 1/2, 1, 3/2 …..
For 11H,136C,3115P: I = 1/2
For 21H, 147N I = 1
(nonspherical charge distribution: electric quadrupole)
number of states in magnetic field 2I+1
In a Magnetic Field
DE=(h/2)Bo
Bo is related to strength
of magnetic field
h is Planck’s Constant
DE is in the radio frequency range
Absorbance of RF
In magnetic field spinning
nucleus precesses about
applied magnetic field
(Larmor Frequency)
when same frequency RF
is applied
electric field of radiation
and electric field of
precessing nucleus
couple
E is transferred and spin
changes -Resonance
Relaxation
How is this energy dissipated?
T1 spin-lattice or longitudinal relaxation process
transfer of E from excited protons to surrounding protons
T2 spin-spin transverse relaxation
transfer of E among precessing protons, result is line
broadening
Instrumentation
Magnetic field, radio frequency generator
Instrument
1945-46 at Stanford
Professor Bloch
Nobel Prize 1952
Sample
Typically if want to observe 1H NMR need to avoid
solvent with protons
used deuterated solvent or solvent with no protons
for example: C6D6, CDCl3 or CCl4
sample is held in a 5mm tube typically 2 mg in 0.5 mL)
sample is spun in the magnetic field to average out
field inhomogeneities
Magnets
1953: 1.41 Tessla or 60 MHz for proton resonance
Now: 200-500 MHz magnets are common
as high as 900 MHz in some NMR research Labs
magnetic fields are large:
in the case of 500 MHz magnetic
5Gauss lines forma a
15 ft sphere about the magnets
Modern Instrument
Chemical Shift
Electron density in a magnetic field circulates generating
a magnetic field in opposition to the applied field
thus shielding the nucleus….
Since electron density for each type of proton
environment is different get different resonance
absorption of RF
eff = (g/2)Bo(1-s) s is the shielding constant
reference position relative
to the standard TMS
tetramethylsilane
H3C
Si
H3C
CH3
CH3
NMR Scale
Set TMS to zero Hz (300 MHz magnet)
3000 Hz
0 Hz
if we use this scale must specify the strength of magnet
as frequency of resonance will change with field
better to use dimensionless units: d (ppm)
freq/applied field x 106 = d
10 ppm
0 ppm
NMR Scales
3000 Hz
300 MHz
0 ppm
10 ppm
6000 Hz
10 ppm
0 Hz
600 MHz
0 Hz
0 ppm
Field Strength Effect
Hb
Hx
60 MHz
Ha
CN
300 MHz
Chemical Shifts
As the shift depends somewhat on electron density
electronegativity may be a guide for chemical shifts
electron density around protons of TMS is high
positive d increases to left of TMS
increase d means deshielded relative to TMS
since C is more electronegative than C expect:
R3CH>R2CH2>RCH3>CH4
1.6
1.2
0.8
NMR Scales
3000 Hz
300 MHz
0 Hz
0 ppm
10 ppm
Higher frequency-less shielded
Lower frequency-more shielded
6000 Hz
10 ppm
600 MHz
0 Hz
0 ppm
Acetylene
based on electronegativity expect higher chemical shift
than ethylene
Apparent anomaly H-CC chemical shift is 1.8 ppm
WHY?
linear molecule: if aligned with magnetic field
then -electrons can circulate at right angles to
field and generate magnetic field in opposition to
applied field thus: protons experience diminished
field and thus resonance at lower frequency
than expected
1.7-1.8 ppm
Aldehydes
Deshielded position of aldehyde proton
observed at 9.97 ppm (acetaldehyde)
Benzene
Ring current effect deshields aromatic protons
7.0-8.0 ppm (depending on substitution)
[18]Annulene
H
H
H
Outside protons are
deshielded 9.3 ppm
H
H
H
H
H
protons on inside
shielded -3.0 ppm
H
H
H
H
H
H
H
H
H
H
Acetophenone
All protons are deshielded due tp ring currents
Ortho-protons are further
deshielded due to carbonyl
meta, para 7.40 ppm
ortho 7.85 ppm
Ring current effect infer planarity and aromaticity
General Regions of Chemical Shifts
alkyne
monosubstituted aliphatic
disubstituted aliphatic
alkene
aldehydic
10
9
8
7
6
Aromatic
5
4
3
2
1
0
ppm
Integration: Benzyl Acetate
Integration 5:2:3
At high resolution see multiplet
Spin-spin Coupling
Chemically inequivalent protons: field of one proton
affects the other
normally only see up to 3-bond coupling
-1/2
-1/2
OR
+1/2
RO
H
+1/2
H
Spin-spin Coupling
Each proton has a unique absorption but effected by
magnetic field of other proton
J is the coupling constant
OR
RO
H
H
Coupling
C-H sees CH2 protons
CH2 sees C-H proton
(+1, 0, -1)
(+1/2, -1/2)
OR
H
H
H
Ethylbenzene
Typical ethyl pattern
A2B3
quartet
triplet
Pascal’s Triangles
Isopropylbenzene
Ethanol in CDCl3
Rapid exchange of OH: do not see coupling
CH3CH2OH
Ethanol in DMSO
No exchange
CH3CH2OH
Doublet of Quartets
CH3CH2OH
Can see:
J(CH2-OH) and
J(CH3-CH2)
N-methylcarbamate
14N
has I =1, if exchange is rapid no coupling
intermediate or slow --broad NH;
O
N
H
O
H-C-N-H Coupling
In trifluoroacetic acid, amine is protonated
see methylene coupling to N-H protons
Fluoroacetone, CH3COCH2F
19F
has I = 1/2
J2
J4
Other Magnetic Heteroatoms
2H
(Deuterium): I = 1;
simplifies proton spectrum as H-D coupling is small
X-CH2-CH2-CH2-COY
X-CH2-CH2-CD2-COY
triplet, quintet, triplet
triplet, slightly broad triplet
31P:
I = 1/2 (100% natural abundance)
large coupling constants P-H 200-700 Hz
29Si:
I = 1/2 (4.7% Natural abundance)
Si-CH 6 Hz; low intensity (satellites)
13C:
I = 1/2 (1.1% Natural abundance)
not seen unless enriched with 13C
Chemical Shift Equivalence
Nuclei are chemical shift equivalent if they are
interchangeable through a symmetry operation or
by a rapid process.
Rotation about a simple axis (Cn)
Reflection through a plane of symmetry (s)
Inversion through a center of symmetry (i)
Rotation and Reflection
C2 axis of rotation Environments are indistinguishable
H
H
Cl
Cl
Reflection through a plane; protons are mirror images
of each other (enantiotopes)
H
Cl
H
F
H
H3C
H
CO2H
Enantiotopes and Diastereotopes
Enantiotopic by i
H
Cl
H
H3C
Cl
H
H
H
Methylenes are diastereotopic
not equivalent
H3C
couple to each other
CH3
H
CO2H
HO
H
Chiral moelcule
Diastereotopic protons
(achiral molecule)
Plane makes H1’s and H2’s
equivalent
HO2C
H1
no plane through CH2’s
thus the protons are diastereotopic
H2
HO
H2
H1
CO2H
H
Diastereotopic protons can not be placed in same
chemical environment
Rapid Exchange
At high T see an average spectrum
Equilibrium at low T
13C
NMR Spectroscopy
12C
not magnetically active but
13C has I = 1/2
Natural abundance is 1.1%
sensitivity is 1/5700 of 1H
this problem is overcome with Fourier Transform (FT)
NMR instrumentation (1970’s)
use broadband decoupling of protons
so see no coupling and get NOE enhancement in
signal intensity
13C
13C{1H}
NMR
13C
samples usually run in CDCl3 and chemical
shifts are reported relative to TMS
300 MHz for 1H NMR == 75.5 MHz for 13CNMR
10 mg in 0.4 mL of solvent in 5 mm tube
13C
NMR of diethylphthalate
Proton coupled
13C{1H}
NMR of diethylphthalate
Proton decoupled
13C{1H}
NMR of diethylphthalate
Proton decoupled
10-s delay
Peak Intensity
in 13C NMR the relaxation times vary over a wide
range so peak areas do not integrate for the
correct number of nuclei
long delays could work but the time required is
prohibitive
NOE response is not uniform for all C atom
environments
C atoms without protons attached give low intensity
Deuterium Substitution
Substitution of D for H results in decreased intensity
deuterium has I = 1 so 13C is split into 3 lines
ratio 1:1:1
possible spin states for D are -1, 0 +1
thus CDCl3 exhibits a 1:1:1 triplet in 13C NMR
Chemical Shifts
Carbon chemical shifts parallel (generally) proton shifts
but with a much broader range
eg. Two substituents on a benzene ring
para: three carbon peaks
meta: four peaks
R
ortho: three peaks
R
R
R
R
R
t-butyl alcohol
2,2,4-trimethyl-1,3-pentanediol
Alkenes, Alkynes and Aromatics
Alkenes: sp2 carbons seen in range 110-150 ppm
Alkynes: sp carbons seen in range 65-95 ppm
Aromatic: benzene 128.5 ppm
substituted +/-35ppm
substituted carbons decreased peak height
longer T1 and diminished NOE
Carbon based Functional Groups
Ketones: R2CO 203.8 ppm(acetone)
Aldehydes: RHCO 199.3 ppm (Acetylaldehyde)
Carboxylic acids: RCO2H 150-185 ppm
Nitriles: RCN 150-185 ppm
Oximes: R2CN(OH) 145-165 ppm
Example
HO
OH
N
N
159.2
H3C
11.50
C
H2
29.00
CH3
11.00
158.7
H3C
18.75
C
H2
CH3
21.50
9.75
13C-1H
Coupling
Coupling is less important than in 1H NMR
since routinely decoupled.
One-bond C-H coupling: 110-320 Hz
two bond: -5 to 60 Hz
three bond: about same as two bond for sp3 C
but for aromatics three bond is often
bigger than two bond
in Benzene: 3JC-H = 7.4 Hz, 2JC-H = 1.0 Hz
Example Spectra 1: C5H10O
O
C
CH
H3C
CH3
quartet
H3C
Singlet: 211.8 ppm
doublet
Example Spectra 2: C4H10O
doublet
OH
quartet
triplet
CH
H3C
C
H2
CH3
Example Spectra 3: C11H14O2
O
doublet
C
H2C
H2
C
O
triplet
singlet
CH3
C
H2
quartet
Other Nuclei for NMR
Nuclei
2H
6Li
15N
19F
23Na
29Si
31P
Spin
(1)
(1)
(1/2)
(1/2)
(3/2)
(1/2)
(1/2)
Nat. Abund.
0.015
7.42
0.37
100
100
4.7
100
19F
NMR Spectrum of fluoracetone
19F
NMR: Fluoracetophenone
29Si
NMR Spectrum of TMS
29Si
NMR:triethylsilane
29Si
NMR:1,1,3,4tetramethyldisiloxane
31P
NMR Spectrum of H3PO4
31P
NMR Spectrum
Ph
Ph
P
Cl
Rh
N
H3C
(Solvent)
CH3
H3C
Ph
Ph
P
(Solvent)
Rh
N
H3C
H3C
CH3
Cl
31P
NMR
PPh2
CH3
Pt
PPh2
Cl
31P
NMR
PPh2
CH3
Pt
PPh2
PR3
+
Diastereomers
PPh2
CH3
Pt
PPh2
PRR'R''
+