Chapter 5: The Structure and Function of Large Biological Molecules
advertisement

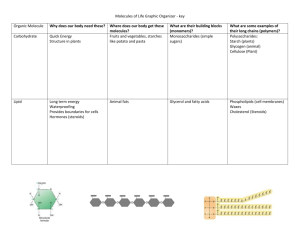
Chapter 5: The Structure and Function of Large Biological Molecules Overview: The Molecules of Life • All living things are made up of ________________ of large biological molecules: _______________________________________________ • Within cells, small organic molecules are joined together to form larger molecules • _____________________ are large molecules composed of thousands of covalently connected atoms (They include: carbohydrates, proteins and nucleic acids) • Molecular ______________ and ________________ are inseparable Concept 5.1: Macromolecules are polymers, built from monomers • A ________________ is a long molecule consisting of many __________________________ • These small building-block molecules are called __________________ • Three of the four classes of life’s organic molecules are polymers: – ________________________ – ________________________ – ________________________ • The Synthesis and Breakdown of Polymers – All polymers are built by ______________________________. When this happens, the two monomers are covalently bonded to each other through the _________________________________. This is called a condensation reaction. • A ____________________________ or more specifically a ____________________________ occurs when two monomers bond together through the loss of a water molecule • Each monomer contributes part of the water molecule: one gives the (OH) while the other gives the (-H) • ______________ are macromolecules that speed up the dehydration process. – Polymers are disassembled to monomers by ________________, a reaction that is essentially the reverse of the dehydration reaction • Hydrolysis means to _______________________. Bonds of the monomer are broken by the addition of water. The water molecule splits. • One monomer gets the (-OH); the other gets the (-H) – Digestion is an example of hydrolysis in our body. – Cells later use dehydration to reassemble new polymers • The Diversity of Polymers – Each cell has _____________________________________ of macromolecules – Macromolecules vary among cells of an organism, vary more within a species, and vary even more between species – An immense variety of polymers can be built from a small set of monomers (there are only 40-50 common monomers) – The key to diversity is the __________________________________ of the monomers. Concept 5.2: Carbohydrates serve as fuel and building material • _____________________ include sugars and the polymers of sugars • The simplest carbohydrates are _____________________, or single sugars • _____________________ are double sugars • Carbohydrate macromolecules are _______________________, polymers composed of many sugar building blocks • Sugars – ____________________ have molecular formulas that are usually multiples of ____________ – ______________ (C H O ) is the most common monosaccharide 6 12 6 – Monosaccharides are classified by • The location of the ___________________ (as ___________ or _____________) • The number of carbons in the carbon skeleton (3, 5 and 6 carbons are most common) – Though often drawn as _______________________, in aqueous solutions many sugars ________________ – Monosaccharides serve as a major fuel for cells and as raw material for building molecules – A ____________________ is formed when a ______________________ joins two monosaccharides – This covalent bond is called a ________________________ • Examples: sucrose (table sugar = glucose + fructose); maltose (brewing sugar= glucose + glucose); lactose (milk sugar = glucose + galactose) – Polysaccharides • _________________, the polymers of sugars, have storage and structural roles. • They are macromolecules with a few hundred to a few thousand monosaccharides joined by glycosidic linkages. • The structure and function of a polysaccharide are determined by its sugar monomers and the positions of glycosidic linkages • Some function for ___________; others for _____________ • Storage Polysaccharides – ____________, a ________________________________, consists entirely of __________________________ • Plants store surplus starch as granules within chloroplasts and other _____________ • Making starch allows a plant to store energy • The bonds holding the starch together are broken by hydrolysis to release glucose; the major cellular fuel – _________________ is a storage polysaccharide in animals • Humans and other vertebrates store glycogen mainly in __________ and ___________________ • Hydrolysis of glycogen releases glucose when needed • The stored glycogen is quickly depleted and must be replenished daily (a draw-back of low carb diets) • Structural Polysaccharides – The polysaccharide _______________ is a major component of the _____________________________ • Like starch, cellulose is a polymer of glucose, but the glycosidic linkages differ • The difference is based on two ring forms for glucose: alpha () and beta () • Polymers with glucose are _______________ • Polymers with glucose are _______________ – In straight structures, H atoms on one strand can hydrogen-bond with OH groups on other strands – Parallel cellulose molecules held together this way are grouped into ________________, which form strong building materials for plants – Enzymes that digest starch by hydrolyzing linkages __________ hydrolyze linkages in cellulose. This makes cellulose _________________________. – Cellulose in human food passes through the digestive tract as _______________________ – Some ___________________ use enzymes to digest cellulose – Many herbivores, from cows to termites, have symbiotic relationships with these microbes – _______________, another structural polysaccharide, is found in the __________________________. It is similar to cellulose except that it has a nitrogen-containing appendage. – Chitin also provides structural support for the __________________ ____________ Concept 5.3: Lipids are a diverse group of hydrophobic molecules • _________________ are the one class of large biological molecules that ____________________________ • The unifying feature of lipids is having ____________________________ • Lipids are ___________________ because they consist mostly of hydrocarbons, which form ______________________________ • The most biologically important lipids are ________, _______________, and ________________ • Fats – _________ are constructed from two types of smaller molecules: _________________________ • _______________ is a _____________________ with a ______________________ attached to each carbon • A ______________ consists of a _______________________ attached to a _____________________________ ( usually 16-18 carbons in length). • They are joined as a result of a dehydration reaction. – To make the fat molecule, three fatty acid molecules each join to glycerol by an ester linkage (a bond between a hydroxyl group and a carboxyl group) – The resulting fat is called a _________________ (i.e _____________) ***Note: the fatty acids can all be the same, or they can be two or three different kinds. – Fats separate from water because water molecules form hydrogen bonds with each other and exclude the fats – Fatty acids vary in length (number of carbons) and in the number and locations of double bonds – ________________________ have the maximum number of hydrogen atoms possible and no double bonds • Saturated fats are solid at room temp. and include most animal fats. – ________________________ have one or more double bonds • Unsaturated fats are liquid at room temp. and include oils, plant fats and fish fats. – A diet rich in saturated fats may contribute to cardiovascular disease through plaque deposits – ________________________ is the process of converting unsaturated fats to saturated fats by adding hydrogen (Ex: peanut butter and margarine—keeps the oils from settling out) – Hydrogenating vegetable oils also creates unsaturated fats with ___________________________ – These _______________ may contribute more than saturated fats to cardiovascular disease. They are common in baked goods and processed foods. – The major function of fats is _____________________ • One gram of fat stores _____________ as much energy as a gram of starch – Humans and other mammals store their fat in _________________. • These cells ___________________ as fat is either deposited or withdrawn – Adipose tissue also ________________________________________ (subcutaneous fat) • Phospholipids – In a ___________________, two fatty acids and a phosphate group are attached to glycerol – The two fatty acid ________________________, but the phosphate group and its attachments form a _____________________ – When phospholipids are added to water, they self-assemble into a __________, with the hydrophobic tails pointing toward the interior – The structure of phospholipids results in a _____________________ __________________________ – Phospholipids are the major component of all cell membranes • Steroids – ____________ are lipids characterized by a carbon skeleton consisting of four fused rings. They include many different types of hormones. – _________________, an important steroid, is a _________________ ________________________. It is synthesized in the liver. – Although cholesterol is essential in animals, ____________________ may contribute to cardiovascular disease Concept 5.4: Proteins have many structures, resulting in a wide range of functions • _____________—comes from the Greek word ____________, meaning first place. They are super important to living organisms. • Proteins account for more than ______ of the dry mass of most cells • Protein functions include _______________________________________ ____________________________________________________________ • _____________ are a type of protein that acts as a ________________ to speed up chemical reactions – Life would not be possible without enzymes. They regulate metabolism. – Enzymes can perform their functions _______________, functioning as workhorses that carry out the processes of life • Polypeptides – ________________ are polymers built from the same set of ____________________ – A ____________ consists of one or more polypeptides • Amino Acid Monomers – _______________ are organic molecules with __________________ ____________ – Amino acids ____________ in their properties due to differing side chains, called ___________________ • At the center of the amino acid is an _____________________ called the ____________________. • Its four partners are: an amino group, a carboxyl group, a hydrogen atom, and an R group. • Amino Acid Polymers—how are amino acids linked to form polymers? – Amino acids are linked by _____________________ • These bonds form between the ___________________ of one amino acid and the ____________________ of the other during a __________________________. Repeated many times, this results in a _____________________. – A polypeptide is a ______________________________. At one end is a free amino group (N-terminus); at the other is a free carboxyl group (C-terminus) – Polypeptides range in length from a few to more than a thousand monomers – Each polypeptide has a unique linear sequence of amino acids • Protein Structure and Function – A functional protein is not just a polypeptide chain. It consists of one or more polypeptides twisted, folded, and coiled into a unique shape – The sequence of amino acids determines a protein’s three-dimensional structure – A protein’s structure determines its function – Four Levels of Protein Structure • The ________________________ of a protein is its ____________________________________ • ________________________, found in most proteins, consists of _________________ in the _______________________ • ______________________ is determined by interactions among various __________________ (R groups) • ______________________ results when a protein consists of _________________________________ – __________________, the sequence of amino acids in a protein, is like the order of letters in a long word • Primary structure is determined by ______________________ ______________________ – The coils and folds of ________________________ result from ____________________between repeating constituents of the polypeptide backbone • Typical secondary structures are a ________ called an _______ and a _______________ called a ___________________ – ____________________ is determined by interactions between R groups, rather than interactions between backbone constituents • These interactions between R groups include ______________ ___________________________________________________ ___________________________________________________ • Strong covalent bonds called ____________________ may reinforce the protein’s structure – ______________________ results when two or more polypeptide chains form one macromolecule • _______________ is a fibrous protein consisting of three polypeptides coiled like a rope • _______________ is a globular protein consisting of four polypeptides: two alpha and two beta chains – Sickle-Cell Disease: A Change in Primary Structure • A slight change in ___________________ can affect a protein’s structure and ability to function – Sickle-cell disease, an inherited blood disorder, results from a ______________________________ in the protein hemoglobin – What Determines Protein Structure? • In addition to primary structure, physical and chemical conditions can affect structure • Alterations in pH, salt concentration, temperature, or other environmental factors can cause a _____________________ • This loss of a protein’s native structure is called _____________ • A denatured protein is _________________________ – Protein Folding in the Cell • It is hard to predict a protein’s structure from its primary structure • Most proteins probably go through several states on their way to a stable structure • ________________ are ___________________ that assist the _______________________________ • Scientists use _________________________ to determine a protein’s structure • Another method is ___________________________ (NMR) spectroscopy, which does not require protein crystallization • Bioinformatics uses computer programs to predict protein structure from amino acid sequences Concept 5.5: Nucleic acids store and transmit hereditary information • The amino acid sequence of a polypeptide is programmed by a unit of inheritance called a ___________ • Genes are made of DNA, a ______________________ • There are two types of nucleic acids: – _____________________________ – _____________________________ • DNA provides directions for its own replication • DNA directs synthesis of ____________________________ and, through mRNA, ______________________________ • Protein synthesis occurs in __________________ • The Structure of Nucleic Acids – Nucleic acids are polymers called ___________________ – Each polynucleotide is made of monomers called ________________ – Each nucleotide consists of a ________________________________ _____________________________________ – The portion of a nucleotide without the phosphate group is called a __________________ – Nucleotide Monomers • Nucleoside = nitrogenous base + sugar • There are two families of nitrogenous bases: o ______________________ (cytosine, thymine, and uracil) have a single six-membered ring o _________________ (adenine and guanine) have a six-membered ring fused to a five-membered ring • In DNA, the sugar is _________________; in RNA, the sugar is ______________ • ___________________ = nucleoside + phosphate group – Nucleotide Polymers • Nucleotide polymers are linked together to build a polynucleotide • Adjacent nucleotides are joined by ___________________ that form between the ______________________________________ ______________________________________________________ • These links create a backbone of sugar-phosphate units with nitrogenous bases as appendages • The _____________________ along a DNA or mRNA polymer is ____________ for each gene – The DNA Double Helix • A DNA molecule has two polynucleotides spiraling around an imaginary axis, forming a __________________ • In the DNA double helix, the two backbones run in __________________________ directions from each other, an arrangement referred to as ___________________ • One DNA molecule includes many genes • The nitrogenous bases in DNA pair up and form hydrogen bonds: ______________________________________________________ – DNA and Proteins as Tape Measures of Evolution • The linear sequences of nucleotides in DNA molecules are ___________________________________________________ • Two closely related species are more similar in DNA than are more distantly related species • Molecular biology can be used to assess _____________________ – The Theme of Emergent Properties in the Chemistry of Life: A Review • Higher levels of organization result in the emergence of new properties • Organization is the key to the chemistry of life You should now be able to: 1. List and describe the four major classes of molecules 2. Describe the formation of a glycosidic linkage and distinguish between monosaccharides, disaccharides, and polysaccharides 3. Distinguish between saturated and unsaturated fats and between cis and trans fat molecules 4. Describe the four levels of protein structure • You should now be able to: 5. Distinguish between the following pairs: pyrimidine and purine, nucleotide and nucleoside, ribose and deoxyribose, the 5 end and 3 end of a nucleotide